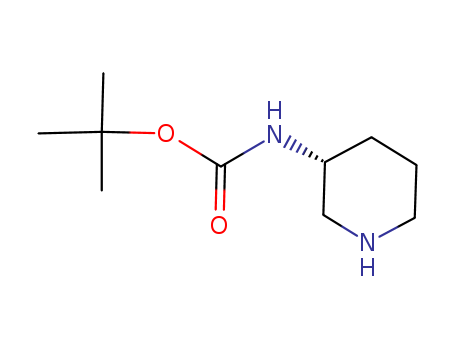
309956-78-3
- Product Name:(R)-3-Boc-aminopiperidine
- Molecular Formula:C10H20N2O2
- Purity:99%
- Molecular Weight:200.281
Product Details
pd_meltingpoint:121.0 to 125.0 °C
Manufacturer supply (R)-3-Boc-aminopiperidine 309956-78-3 with sufficient stock and high standard
- Molecular Formula:C10H20N2O2
- Molecular Weight:200.281
- Vapor Pressure:0.000854mmHg at 25°C
- Melting Point:121.0 to 125.0 °C
- Refractive Index:1.479
- Boiling Point:304.8 °C at 760 mmHg
- PKA:12.37±0.20(Predicted)
- Flash Point:138.2 °C
- PSA:50.36000
- Density:1.02 g/cm3
- LogP:1.98280
(R)-3-(Boc-Amino)piperidine(Cas 309956-78-3) Usage
|
Solubility in organics |
Soluble in methanol and ethanol. |
|
storage |
Stable under recommended storage conditions. Incompatible with oxidizing agents. |
|
Chemical Composition and Structure |
White to off-white solid with specific optical activity. The chemical structure involves a Boc-protected amino group on the piperidine ring. |
|
Category and Use |
Originated as a synthetic intermediate in pharmaceutical research and development. Developed as a key component of antidiabetic medications like linagliptin. Common organic synthesis intermediate and medicinal chemistry intermediate. Primarily used for the synthesis of drug molecules and biologically active molecules, especially inhibitors of dipeptidyl peptidase-4 (DPP-4), such as alogliptin and linagliptin. Also a key synthetic intermediate for the drug molecule linagliptin tablets, which lower blood sugar. Inhibits DPP-4, an enzyme involved in glucose regulation, leading to lower blood sugar levels. |
|
Production Methods |
Two main process routes: one using d-glutamic acid as starting material and the other using 3-hydroxypiperidine. Each route involves several steps including esterification, reduction, and protection reactions. |
InChI:InChI=1/C10H20N2O2/c1-10(2,3)14-9(13)12-8-5-4-6-11-7-8/h8,11H,4-7H2,1-3H3,(H,12,13)/t8-/m1/s1
309956-78-3 Relevant articles
Convenient synthesis of (R)-3-[(tert -Butoxycarbonyl)amino]piperidine and (R)-3-[(tert -Butoxycarbonyl)amino]azepane
Kadyrov, Renat,Tok, Oleg L.
, p. 3573 - 3577 (2021/07/25)
(R)-3-[(tert -Butoxycarbonyl)amino]piper...
Synthesis method of chiral 3-aminopiperidine and derivatives of 3-aminopiperidine
-
Paragraph 0135-0136, (2019/09/17)
The invention relates to a synthesis met...
(R)- 3 - Boc - amino piperidine preparation method
-
Paragraph 0064; 0065; 0066; 0067; 0068; 0069; 0070, (2018/04/03)
The invention discloses a preparation me...
Amino-protected (R) - 3-amino-piperidine preparation method
-
Paragraph 0062-0064, (2017/03/18)
The invention discloses a preparation me...
309956-78-3 Process route
-
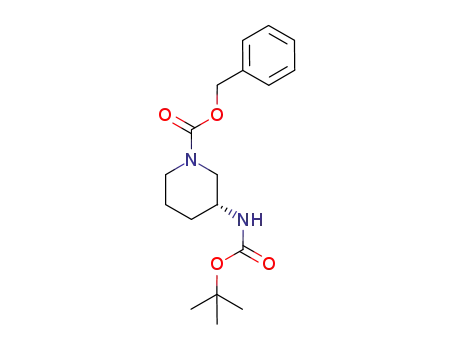
-
485820-12-0
(R)-3-(tert-butoxycarbonylamino)piperidine-1-carboxylic acid benzyl ester

-
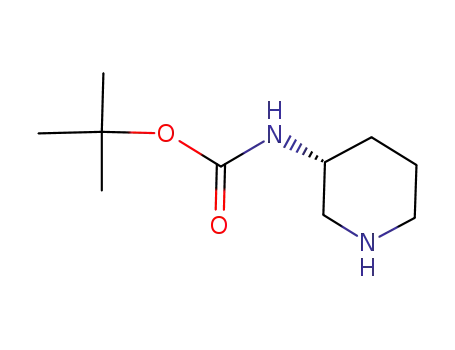
-
309956-78-3,172603-05-3
(R)-piperidin-3-ylcarbamic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol; water;
at 35 - 40 ℃;
for 2h;
under 2250.23 - 3000.3 Torr;
Time;
Autoclave;
Inert atmosphere;
|
95.4% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
at 20 ℃;
for 168h;
under 760.051 Torr;
|
92% |
|
In
ethanol;
|
-
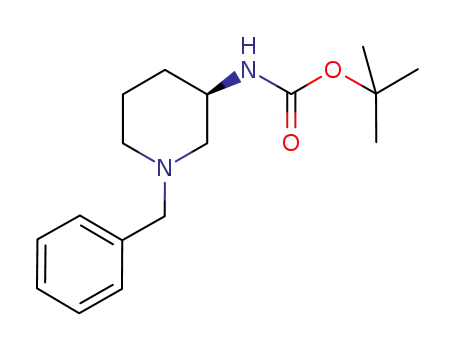
-
454713-13-4,216854-24-9
tert-butyl (R)-(+)-(N-benzylpiperidin-3-yl)carbamate

-

-
309956-78-3,172603-05-3
(R)-piperidin-3-ylcarbamic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 20 - 25 ℃;
for 20h;
under 150015 Torr;
|
97% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 45 ℃;
for 2h;
under 1140.08 Torr;
|
85% |
|
With
hydrogen;
10% palladium on activated carbon;
In
metahnol;
at 20 ℃;
for 24h;
|
309956-78-3 Upstream products
-
485820-12-0

(R)-3-(tert-butoxycarbonylamino)piperidine-1-carboxylic acid benzyl ester
-
454713-13-4

tert-butyl (R)-(+)-(N-benzylpiperidin-3-yl)carbamate
-
1189160-63-1
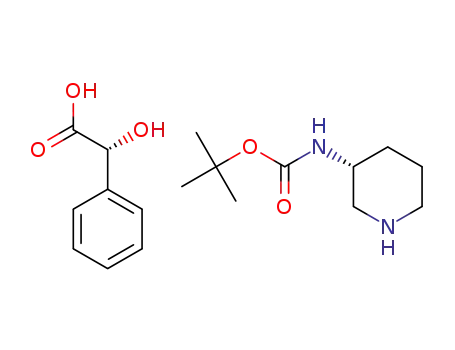
t-butyl (R)-piperidin-3-ylcarbamate R-mandelate
-
6893-26-1
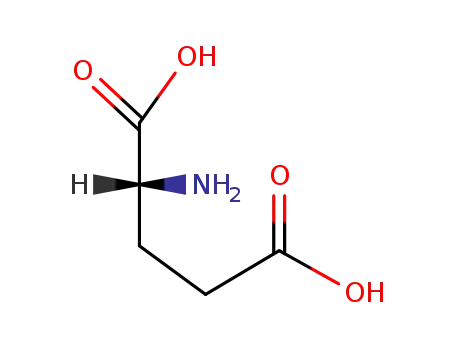
D-Glutamic acid
309956-78-3 Downstream products
-
666816-91-7
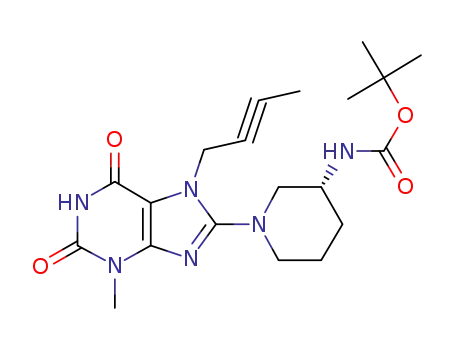
3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)piperidin-1-yl]xanthine
-
868609-89-6
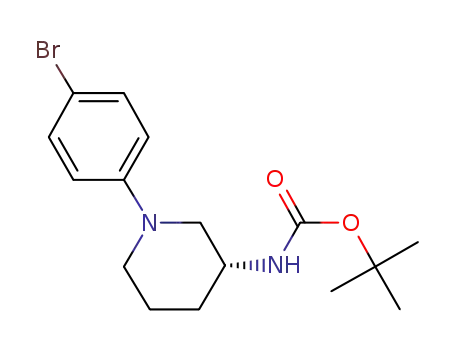
[(R)-1-(4-bromo-phenyl)-piperidin-3-yl]-carbamic acid-tert-butylester
-
853068-38-9
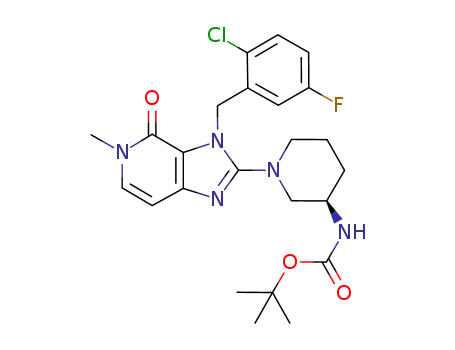
tert-butyl {(3R)-1-[3-(2-chloro-5-fluorobenzyl)-5-methyl-4-oxo-4,5-dihydro-3H-imidazo[4,5-c]pyridin-2-yl]piperidin-3-yl}carbamate
-
853068-43-6
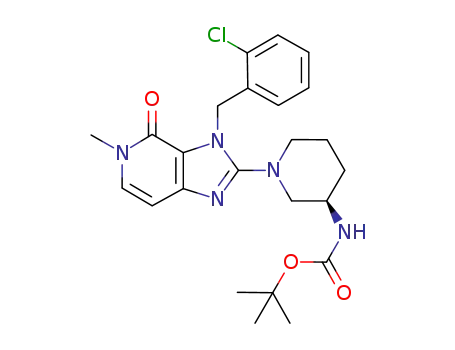
tert-butyl {(3R)-1-[3-(2-chlorobenzyl)-5-methyl-4-oxo-4,5-dihydro-3H-imidazo[4,5-c]pyridin-2-yl]piperidin-3-yl}carbamate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
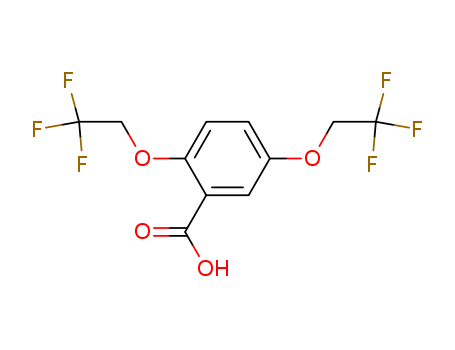
2,5-Bis(2,2,2-trifluoroethoxy)benzoic acid
CAS:35480-52-5
-
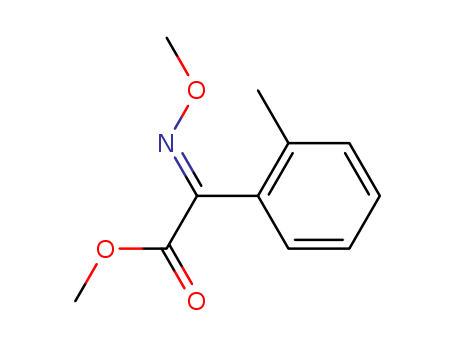
METHYL 2-(METHOXYIMINO)-2-O-TOLYLACETATE
CAS:120974-97-2