188111-79-7
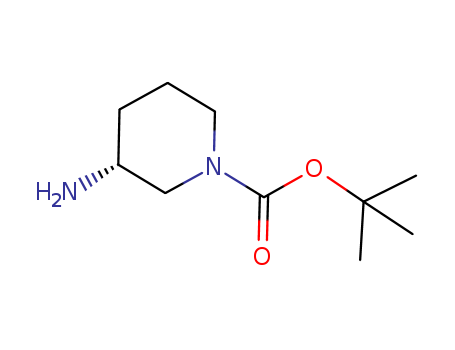
- Product Name:(R)-1-Boc-3-Aminopiperidine
- Molecular Formula:C10H20N2O2
- Purity:99%
- Molecular Weight:200.281
Product Details
Quality manufacturer supply (R)-1-Boc-3-Aminopiperidine 188111-79-7 in stock with high standard
- Molecular Formula:C10H20N2O2
- Molecular Weight:200.281
- Vapor Pressure:0.00456mmHg at 25°C
- Refractive Index:1.4730
- Boiling Point:277.3 °C at 760 mmHg
- PKA:10.35±0.20(Predicted)
- Flash Point:121.5 °C
- PSA:55.56000
- Density:1.041g/cm3
- LogP:1.98280
(R)-1-Boc-3-Aminopiperidine(Cas 188111-79-7) Usage
|
General Description |
(R)-3-Amino-1-Boc-piperidine can be used as a precursor for the preparation of dipeptidyl peptidase IV inhibitors like linagliptin, alogliptin, and other antidiabetic agents. |
InChI:InChI=1/C10H20N2O2/c1-10(2,3)14-9(13)12-6-4-5-8(11)7-12/h8H,4-7,11H2,1-3H3/t8-/m0/s1
188111-79-7 Relevant articles
Asymmetric synthesis of a high added value chiral amine using immobilized ω-transaminases
Petri, Antonella,Colonna, Valeria,Piccolo, Oreste
, p. 60 - 66 (2019)
Chiral N-heterocyclic molecules and in p...
Application of asymmetric hydrogenation in synthesis of Trelagliptin intermediate
-
Paragraph 0014, (2018/09/21)
The invention provides a synthetic metho...
METHOD FOR PRODUCING OPTICALLY ACTIVE 3-AMINOPIPERIDINE OR SALT THEREOF
-
Page/Page column 21, (2010/05/13)
The present invention relates to a metho...
AMIDE DERIVATIVE
-
Page/Page column 120, (2009/12/05)
The present invention relates to a compo...
188111-79-7 Process route
-
-
98977-36-7
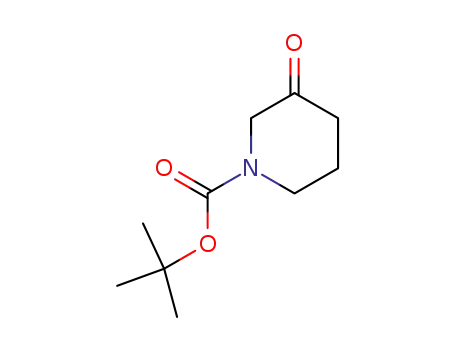
3-oxo-piperidine-1-carboxylic acid tert-butyl ester

-
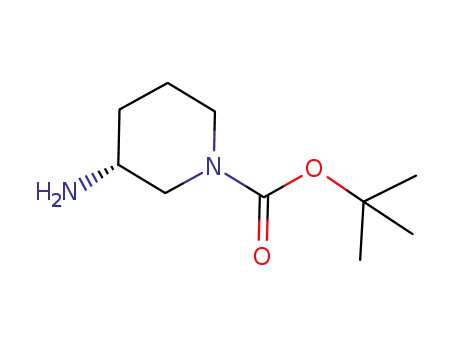
-
188111-79-7
tert-butyl (3R)3-aminopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
pyridoxal 5'-phosphate; immobilized Codex? amine ω-transaminase; isopropylamine;
In
dimethyl sulfoxide;
at 50 ℃;
pH=7.5;
Temperature;
enantioselective reaction;
Enzymatic reaction;
|
70% |
|
Multi-step reaction with 2 steps
1: ammonium acetate / ethanol / 7 h / Reflux; Large scale
2: bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; sodium hydride; C13H18FeP2; hydrogen / dichloromethane / Large scale
With
bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; C13H18FeP2; ammonium acetate; hydrogen; sodium hydride;
In
ethanol; dichloromethane;
|
-
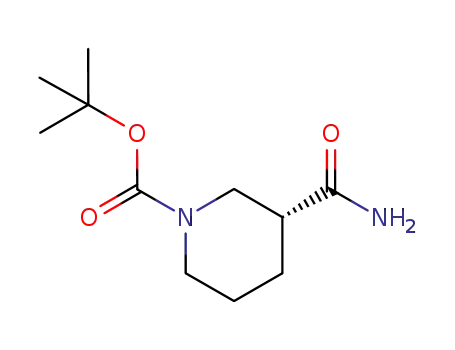
-
915226-43-6
(R)-tert-butyl 3-carbamoylpiperidine-1-carboxylate

-

-
188111-79-7
tert-butyl (3R)3-aminopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
sodium hypochlorite; sodium hydroxide;
In
water;
at 15 - 25 ℃;
for 16h;
|
80% |
188111-79-7 Upstream products
-
320580-76-5
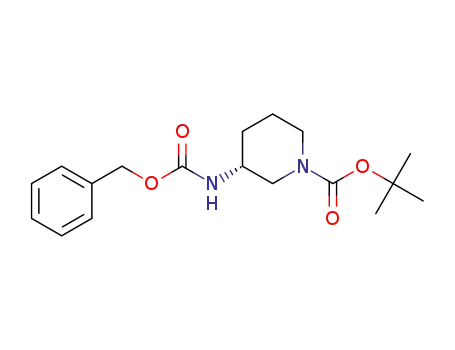
tert-butyl (3R)-3-{[(benzyloxy)carbonyl]amino}piperidine-1-carboxylate
-
915226-43-6

(R)-tert-butyl 3-carbamoylpiperidine-1-carboxylate
-
98977-36-7

3-oxo-piperidine-1-carboxylic acid tert-butyl ester
188111-79-7 Downstream products
-
1009633-72-0
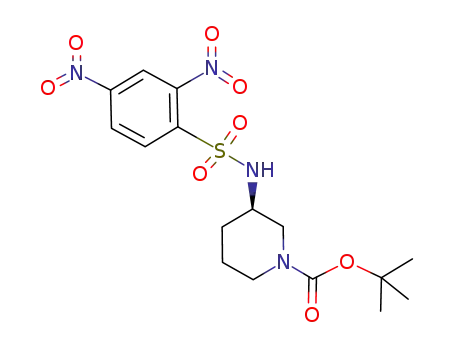
C16H22N4O8S
-
1062132-47-1
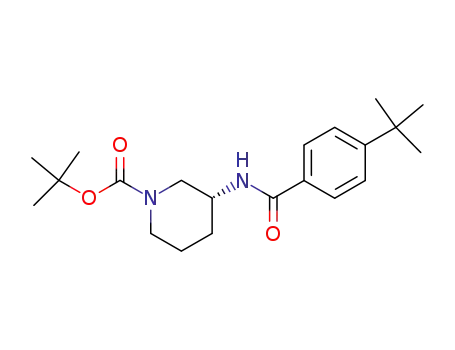
(R)-tert-butyl 3-(4-tert-butylbenzamido)piperidine-1-carboxylate
-
1159996-27-6
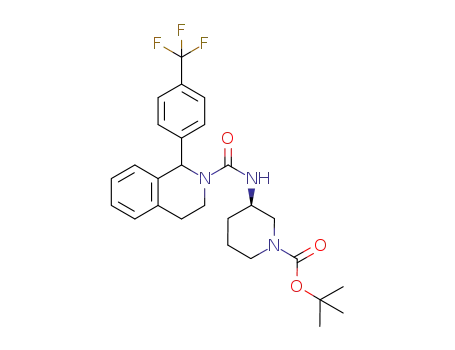
(3R)-tert-butyl 3-(1-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydroisoquinoline-2-carboxamido)piperidine-1-carboxylate
-
1044561-53-6
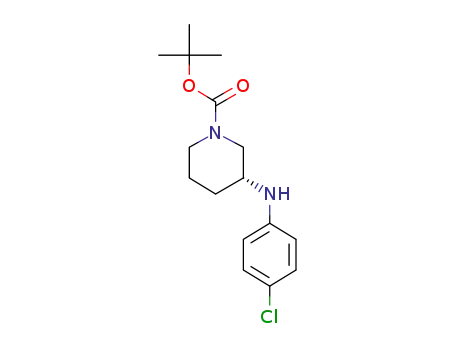
tert-Butyl (3R)-3-[(4-chlorophenyl)amino]piperidine-1-carboxylate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
3,4,5-Trimethoxybenzoic acid methyl ester
CAS:1916-07-0
-
3-(2-Pyrrolidinyl)pyridine
CAS:5746-86-1