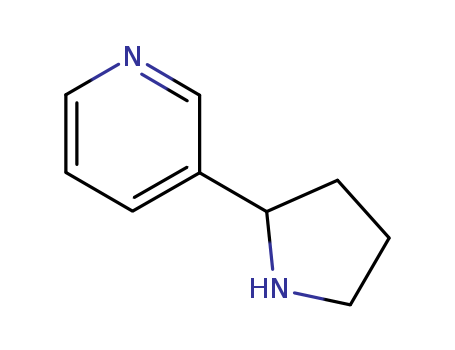
5746-86-1
- Product Name:3-(2-Pyrrolidinyl)pyridine
- Molecular Formula:C9H12 N2
- Purity:99%
- Molecular Weight:148.208
Product Details
pd_meltingpoint:235-236 °C(Solv: ethanol (64-17-5))
Appearance:liquid
Factory Sells Best Quality 3-(2-Pyrrolidinyl)pyridine 5746-86-1 with steady supply
- Molecular Formula:C9H12 N2
- Molecular Weight:148.208
- Appearance/Colour:liquid
- Vapor Pressure:0.00702mmHg at 25°C
- Melting Point:235-236 °C(Solv: ethanol (64-17-5))
- Refractive Index:1.54
- Boiling Point:107-108°C 2mm
- PKA:9.09±0.10(Predicted)
- Flash Point:101°C
- PSA:24.92000
- Density:1,074 g/cm3
- LogP:1.83490
3-(2-Pyrrolidinyl)pyridine(Cas 5746-86-1) Usage
|
Synthesis Reference(s) |
Tetrahedron Letters, 37, p. 1137, 1996 DOI: 10.1016/0040-4039(96)00002-0 |
|
Safety Profile |
Poison by intraperitoneal route.When heated to decomposition it emits very toxic fumesof NOx and HCl. See also NORNICOTINE |
InChI:InChI=1/C9H12N2/c1-3-8(7-10-5-1)9-4-2-6-11-9/h1,3,5,7,9,11H,2,4,6H2/p+1/t9-/m0/s1
5746-86-1 Relevant articles
Preparation method of nicotine and intermediate thereof
-
, (2021/06/02)
The invention relates to a preparation m...
Synthesis method of (R, S-) nicotine
-
Paragraph 0046-0047, (2021/05/05)
The invention belongs to the technical f...
Synthesis method of racemic nicotine
-
Paragraph 0047; 0053-0062, (2021/01/04)
The invention discloses a synthesis meth...
ENANTIOMERIC SEPARATION OF RACEMIC NICOTINE BY ADDITION OF AN O,O'-DISUBSTITUTED TARTARIC ACID ENANTIOMER
-
Page/Page column 8; 17, (2019/07/13)
The present invention relates to a metho...
5746-86-1 Process route
-
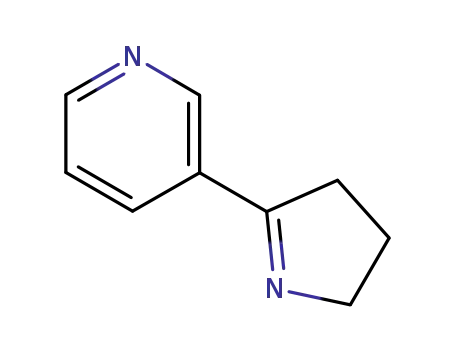
-
532-12-7
Myosmine

-
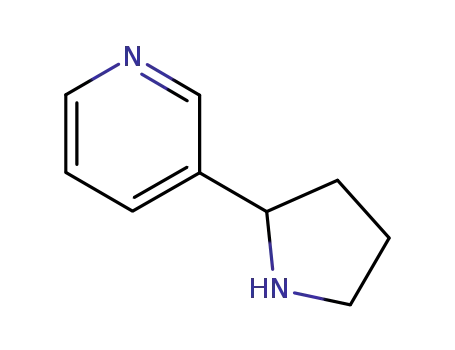
-
5746-86-1,13450-58-3
rac-nornicotine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol;
for 5h;
|
84.7% |
|
With
sodium cyanoborohydride;
In
methanol;
|
68% |
|
With
ethanol; palladium;
Hydrogenation;
|
|
|
With
palladium on activated charcoal; ethanol;
Hydrogenation;
|
|
|
With
sodium tetrahydroborate; acetic acid;
In
methanol;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
for 12h;
Large scale;
|
|
|
Myosmine;
With
acetic acid;
In
methanol;
at -40 ℃;
With
methanol; sodium tetrahydroborate;
at 20 ℃;
for 1h;
|
|
|
With
sodium tetrahydroborate; acetic acid;
In
methanol;
at 20 ℃;
Cooling with ice;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
for 12h;
under 38002.6 Torr;
Reagent/catalyst;
Solvent;
Pressure;
Large scale;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
for 6h;
under 19001.3 Torr;
Pressure;
Solvent;
Reagent/catalyst;
|
|
|
With
sodium tetrahydroborate;
In
isopropyl alcohol;
at 20 ℃;
|
|
|
With
sodium tetrahydroborate; isopropyl alcohol;
at 10 ℃;
Temperature;
|
|
|
With
palladium on activated charcoal; hydrogen;
In
ethanol;
at 50 - 100 ℃;
under 4500.45 Torr;
Temperature;
Pressure;
|
-
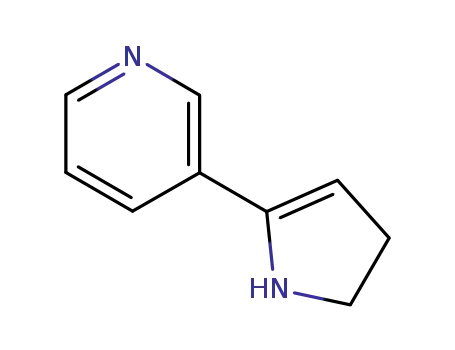
-
1125-96-8
myosmine

-

-
5746-86-1,13450-58-3
rac-nornicotine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol;
for 5h;
under 760.051 Torr;
|
84.7% |
|
at 20 ℃;
Reagent/catalyst;
Inert atmosphere;
|
5746-86-1 Upstream products
-
532-12-7

Myosmine
-
17708-87-1
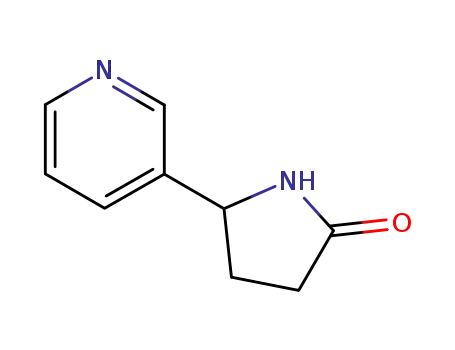
5-(pyridin-3-yl)pyrrolidin-2-one
-
125198-33-6
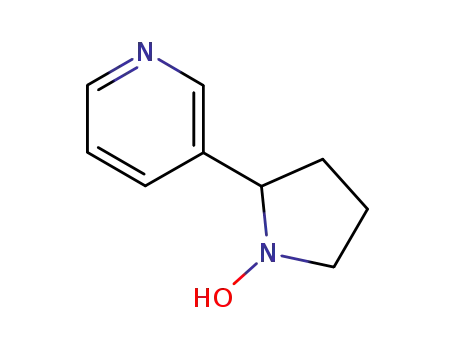
3-(1-Hydroxy-2-pyrrolidinyl)pyridine
-
138084-62-5
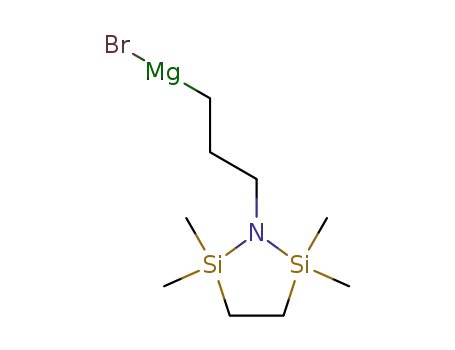
2,2,5,5-tetrametyl-1-aza-2,5-disilacyclopentane-1-propyl magnesium bromide
5746-86-1 Downstream products
-
22083-74-5
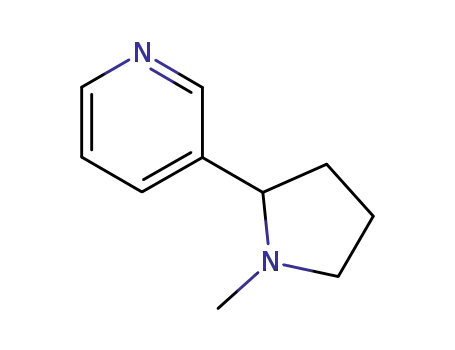
3-(1-methyl-pyrrolidin-2-yl)-pyridine
-
494-97-3
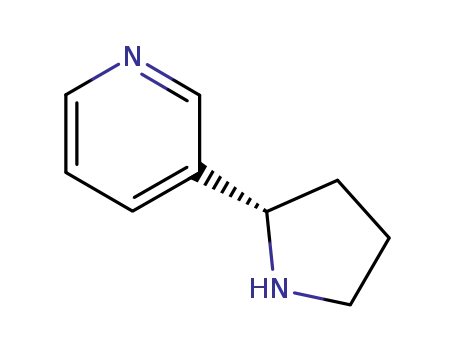
nornicotine
-
7076-23-5
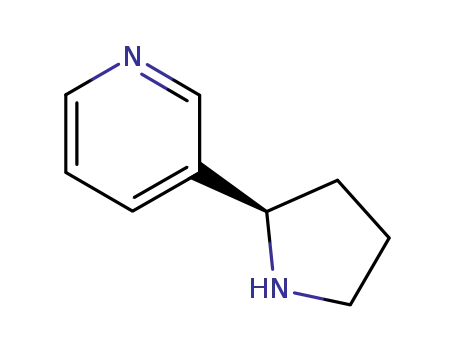
(+)-nornicotine
-
3000-81-5
N'-formylnornicotine
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
(R)-1-Boc-3-Aminopiperidine
CAS:188111-79-7
-
Propyl gallate
CAS:121-79-9