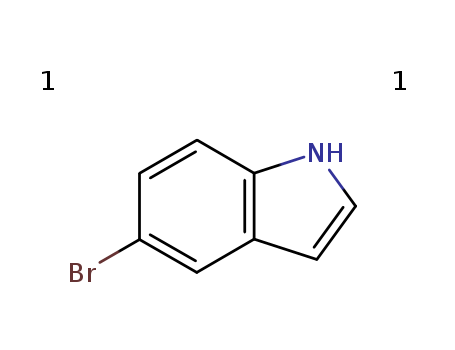
10075-50-0
- Product Name:5-Bromoindole
- Molecular Formula:C8H6BrN
- Purity:99%
- Molecular Weight:264.33
Product Details
pd_meltingpoint:89-92 °C
Appearance:white to light brown powder or chunks
Manufacturer supply top purity 5-Bromoindole 10075-50-0 with ISO standards
- Molecular Formula:C8H6BrN
- Molecular Weight:264.33
- Appearance/Colour:white to light brown powder or chunks
- Vapor Pressure:0.000738mmHg at 25°C
- Melting Point:89-92 °C
- Refractive Index:1.711
- Boiling Point:316.9 °C at 760 mmHg
- PKA:16.04±0.30(Predicted)
- Flash Point:145.5 °C
- PSA:15.79000
- Density:1.66 g/cm3
- LogP:2.93040
5-Bromoindole(Cas 10075-50-0) Usage
|
Purification Methods |
Purify it by steam distillation from a faintly alkaline solution. Cool the aqueous distillate, collect the solid, dry it in a vacuum desiccator over P2O5 and recrystallise it from aqueous EtOH (35% EtOH) or pet ether/Et2O. UV in MeOH has at 279, 287 and 296nm (log 3.70, 3.69 and 3.53). The picrate has m 137-138o(dec) (from max Et2O/pet ether). [UV: Thesing et al. Chem Ber 95 2205 1962, UV and NMR: Lallemand & Bernath Bull Soc Chim Fr 4091 1970, Beilstein 20/7 V 36.] |
InChI:InChI=1/C8H6BrN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
10075-50-0 Relevant articles
Chemoselective deprotection of allylic amines catalyzed by Grubbs' carbene
Alcaide, Benito,Almendros, Pedro,Alonso, Jose M.,Luna, Amparo
, p. 668 - 672 (2005)
A commercially available ruthenium compl...
Ga(DS)3-catalysed double hydroarylation of acetylenic esters with indoles for the synthesis of bisindolyl propanoates
An, Li-Tao,Cai, Jing-Jing,Pan, Xiang-Qiang,Chen, Tang-Ming,Zou, Jian-Ping,Zhang, Wei
, p. 3996 - 3998 (2015)
Abstract An efficient synthetic method f...
CO2-Catalyzed Efficient Dehydrogenation of Amines with Detailed Mechanistic and Kinetic Studies
Riemer, Daniel,Schilling, Waldemar,Goetz, Anne,Zhang, Yu,Gehrke, Sascha,Tkach, Igor,Hollóczki, Oldamur,Das, Shoubhik
, p. 11679 - 11687 (2018)
CO2-catalyzed dehydrogenation of amines ...
Erratum: Integrated structure-based activity prediction model of benzothiadiazines on various genotypes of HCV NS5b polymerase (1a, 1b and 4) and its application in the discovery of new derivatives (Bioorganic and Medicinal Chemistry (2012) 20:7 (2455-2478))
Ismail, Mohamed A.H.,Abou El Ella, Dalal A.,Abouzid, Khaled A.M.,Mahmoud, Amr H.
, p. 5647 - 5647 (2013)
-
Novel Arylindigoids by Late-Stage Derivatization of Biocatalytically Synthesized Dibromoindigo
Schnepel, Christian,Dodero, Veronica I.,Sewald, Norbert
, p. 5404 - 5411 (2021)
Indigoids represent natural product-base...
Hydrophobic Metal Halide Perovskites for Visible-Light Photoredox C?C Bond Cleavage and Dehydrogenation Catalysis
Hong, Zonghan,Chong, Wee Kiang,Ng, Andrew Yun Ru,Li, Mingjie,Ganguly, Rakesh,Sum, Tze Chien,Soo, Han Sen
, p. 3456 - 3460 (2019)
Two-dimensional lead and tin halide pero...
DMSO/t-BuONa/O2-Mediated Aerobic Dehydrogenation of Saturated N-Heterocycles
Cai, Hu,Tan, Wei,Xie, Yongfa,Yang, Ruchun,Yue, Shusheng
, p. 7501 - 7509 (2020)
Aromatic N-heterocycles such as quinolin...
A new and efficient one-pot synthesis of indoles
Bratulescu, George
, p. 984 - 986 (2008)
The synthesis of indoles is accomplished...
Aerobic oxidative dehydrogenation of N-heterocycles over OMS-2-based nanocomposite catalysts: Preparation, characterization and kinetic study
Bi, Xiuru,Tang, Tao,Meng, Xu,Gou, Mingxia,Liu, Xiang,Zhao, Peiqing
, p. 360 - 371 (2020)
OMS-2-based nanocomposites doped with tu...
Dehydrogenation of indoline by cytochrome P450 enzymes: A novel "aromatase" process
Sun, Hao,Ehlhardt, William J.,Kulanthaivel, Palaniappan,Lanza, Diane L.,Reilly, Christopher A.,Yost, Garold S.
, p. 843 - 851 (2007)
Indoline derivatives possess therapeutic...
Two-Dimensional Metal-Organic Layers for Electrochemical Acceptorless Dehydrogenation of N-Heterocycles
Yang, Ling,Ma, Fa-Xue,Xu, Fan,Li, Dong,Su, Liangmei,Xu, Hai-Chao,Wang, Cheng
, p. 3557 - 3560 (2019)
The catalytic acceptorless dehydrogenati...
Synthesis of bromoindole alkaloids from Laurencia brongniartii
Suarez-Castillo, Oscar R.,Beiza-Granados, Lidia,Melendez-Rodriguez, Myriam,Alvarez-Hernandez, Alejandro,Morales-Rios, Martha S.,Joseph-Nathan, Pedro
, p. 1596 - 1600 (2006)
A regioselective synthesis of N-carbomet...
Simple and selective removal of the t-butyloxycarbonyl (Boc) protecting group on indoles, pyrroles, indazoles, and carbolines
Ravinder,Reddy, A. Vijender,Mahesh, K. Chinni,Narasimhulu,Venkateswarlu
, p. 281 - 287 (2007)
A highly selective and efficient deprote...
Luminescent Platinum(II) Complexes with Bidentate Diacetylide Ligands: Structures, Photophysical Properties and Application Studies
Luo, Zaoli,Liu, Yungen,Tong, Ka-Chung,Chang, Xiao-Yong,To, Wai-Pong,Che, Chi-Ming
, p. 2978 - 2992 (2021)
A series of platinum(II) complexes suppo...
Ruthenium-Catalyzed Vinylene Carbonate Annulation by C?H/N?H Functionalizations: Step-Economical Access to Indoles
Li, Bo,Li, Zheyu,Ma, Wenbo,Tan, Yuqiang,Wang, Yang,Yu, Yao,Zhang, Chunran,Zhao, Huan
, (2022/01/11)
A convenient and effective method of rut...
From Tryptophan to Toxin: Nature's Convergent Biosynthetic Strategy to Aetokthonotoxin
Adak, Sanjoy,Lukowski, April L.,Sch?fer, Rebecca J. B.,Moore, Bradley S.
supporting information, p. 2861 - 2866 (2022/02/23)
Aetokthonotoxin (AETX) is a cyanobacteri...
Metal–Organic Layers Hierarchically Integrate Three Synergistic Active Sites for Tandem Catalysis
Quan, Yangjian,Lan, Guangxu,Shi, Wenjie,Xu, Ziwan,Fan, Yingjie,You, Eric,Jiang, Xiaomin,Wang, Cheng,Lin, Wenbin
supporting information, p. 3115 - 3120 (2020/12/09)
We report the design of a bifunctional m...
Visible light mediated selective oxidation of alcohols and oxidative dehydrogenation of N-heterocycles using scalable and reusable La-doped NiWO4nanoparticles
Abinaya, R.,Balasubramaniam, K. K.,Baskar, B.,Divya, P.,Mani Rahulan, K.,Rahman, Abdul,Sridhar, R.,Srinath, S.
, p. 5990 - 6007 (2021/08/24)
Visible light-mediated selective and eff...
10075-50-0 Process route
-
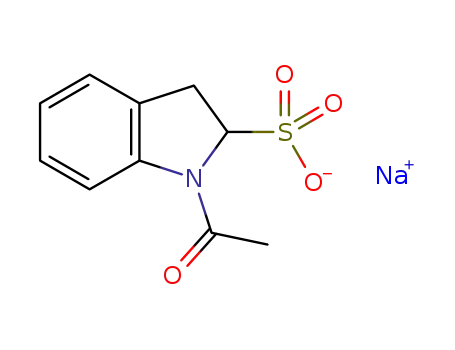
-
26807-69-2
N-acetylindololine-2-sulfonic acid sodium

-
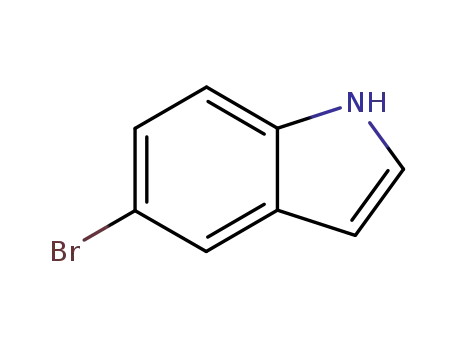
-
10075-50-0
5-bromo-1H-indole
| Conditions | Yield |
|---|---|
|
N-acetylindololine-2-sulfonic acid sodium;
With
bromine;
In
water;
at 0 - 20 ℃;
for 2h;
With
sodium hydroxide;
In
water;
for 20h;
Reflux;
|
92% |
|
N-acetylindololine-2-sulfonic acid sodium;
With
bromine;
In
water;
at 0 - 20 ℃;
With
sodium hydroxide;
In
water;
for 20h;
Reflux;
|
92% |
|
With
bromine;
In
water;
at 0 - 5 ℃;
for 0.5h;
|
88% |
-
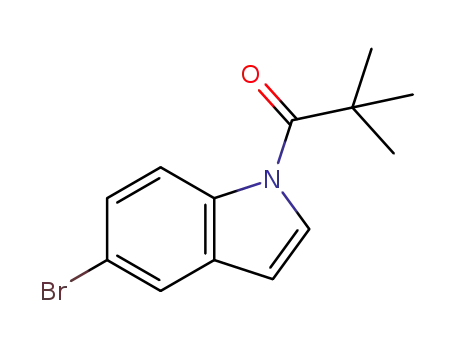
-
1196980-99-0
1-(5-bromo-1H-indol-1-yl)-2,2-dimethylpropan-1-one

-

-
10075-50-0
5-bromo-1H-indole
| Conditions | Yield |
|---|---|
|
With
water; 1,8-diazabicyclo[5.4.0]undec-7-ene;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
99% |
10075-50-0 Upstream products
-
22190-33-6
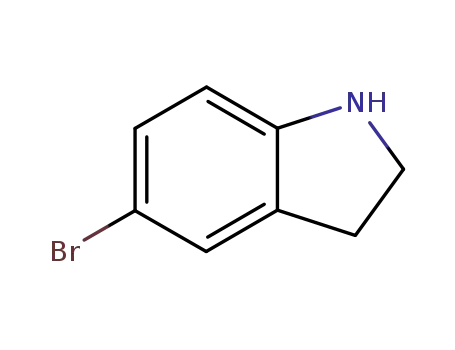
5-bromoindoline
-
7254-19-5
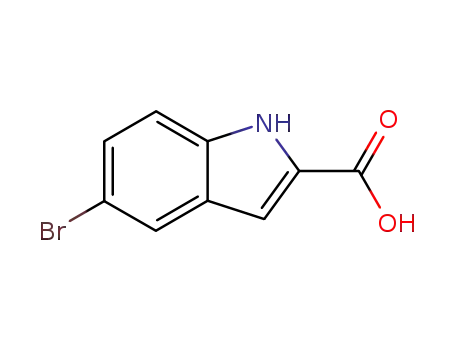
5-bromo-2-indolecarboxylic acid
-
105896-41-1
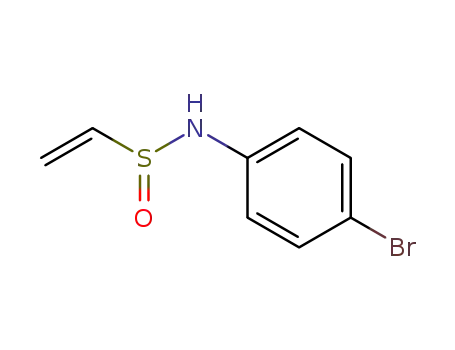
N-(4-bromophenyl)ethenesulfinamide
-
88131-63-9
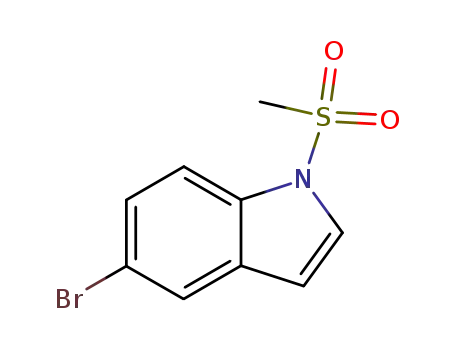
5-Bromo-1-(methylsulfonyl)indole
10075-50-0 Downstream products
-
877-03-2
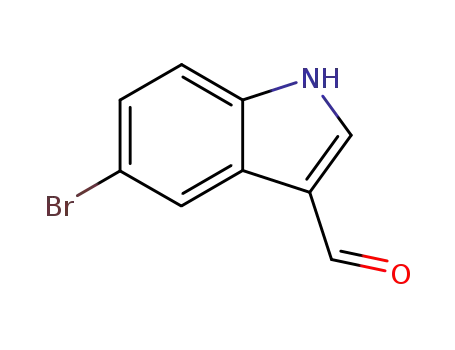
5-bromo-1H-indole-3-carboxaldehyde
-
92557-51-2
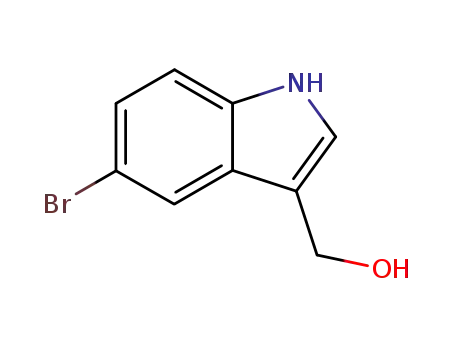
5-Bromo-indole-3-carbinol
-
63843-81-2
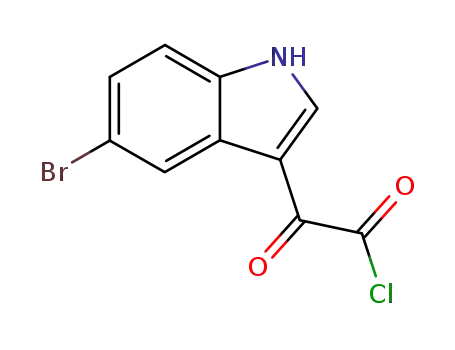
2-(5-bromo-1H-indol-3-yl)-2-oxoacetyl chloride
-
77248-65-8
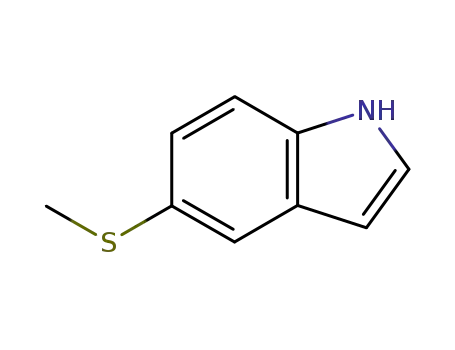
5-methylthio-1H-indole
Relevant Products
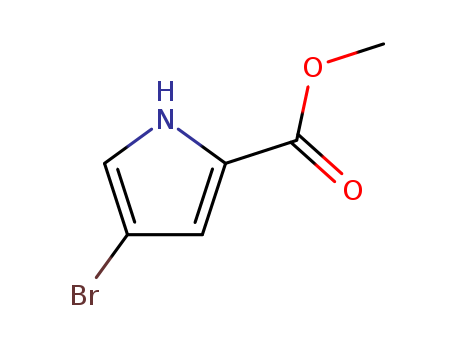
-
4-Bromo-2-(methoxycarbonyl)-1H-pyrrole
CAS:934-05-4
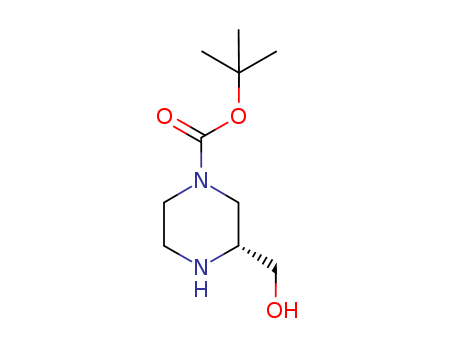
-
(R)-1-Boc-3-hydroxymethylpiperazine
CAS:278788-66-2
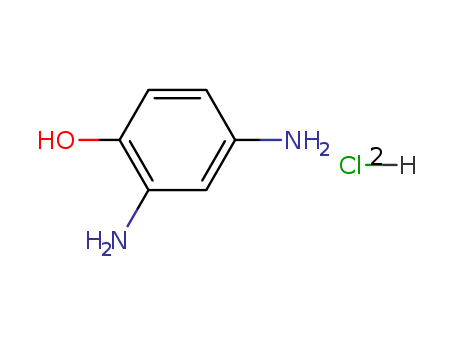
-
2,4-Diaminophenol dihydrochloride
CAS:137-09-7