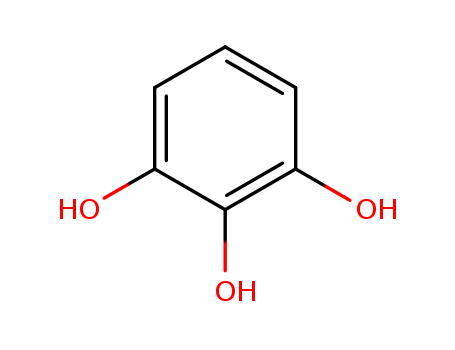
87-66-1
- Product Name:Pyrogallic Acid
- Molecular Formula:C6H6O3
- Purity:99%
- Molecular Weight:126.112
Product Details
pd_meltingpoint:43-47 °C(lit.)
Appearance:white crystalline solid
Factory Supply industrial standard Pyrogallic Acid 87-66-1 In Stock
- Molecular Formula:C6H6O3
- Molecular Weight:126.112
- Appearance/Colour:white crystalline solid
- Vapor Pressure:10 mm Hg ( 167.7 °C)
- Melting Point:43-47 °C(lit.)
- Refractive Index:n20/D 1.387
- Boiling Point:309 °C at 760 mmHg
- PKA:pK1:9.03(0);pK2:11.63(+1) (25°C)
- Flash Point:164.4 °C
- PSA:60.69000
- Density:1.488 g/cm3
- LogP:0.80340
Pyrogallol(Cas 87-66-1) Usage
|
Production Methods |
Pyrogallol is prepared by heating dried gallic acid at about 200°C with the loss of carbon dioxide or by the chlorination of cyclohexanol to tetrachlorocyclohexanone, followed by hydrolysis. |
|
Definition |
ChEBI: A benzenetriol carrying hydroxy groups at positions 1, 2 and 3. |
|
General Description |
Odorless white to gray solid. Sinks and mixes with water. |
|
Air & Water Reactions |
Turns gray on exposure to light or air. Water soluble. |
|
Reactivity Profile |
Pyrogallol is a strong reducing agent. Reacts with alkalis, NH3, antipyrine, camphor, phenol, iron and lead salts, iodine, lime water, menthol and KMnO4. |
|
Hazard |
Toxic by ingestion and skin absorption. |
|
Health Hazard |
The toxic symptoms are similar to those of phenol. It can enter the body by absorption through skin and ingestion. The poisoning effects are nausea, vomiting, gastritis, hemolysis, methemoglobinemia, kidney and liver damage, convulsions, and congestion of lungs. High doses can cause death. Ingestion of 2–3 g of solid can be fatal to humans. The LD50 values varied widely in species. The oral LD50 value in mice is about 300 mg/kg. |
|
Fire Hazard |
Pyrogallol is probably combustible. |
|
Contact allergens |
Pyrogallol belongs to the phenols group. It is an old photograph developer and a low sensitizer in hair dyes. |
|
Biochem/physiol Actions |
Pyrogallol also referred to as 1,2,3-trihydroxybenzene inhibits the response to nitric oxide (NO) in the rat anococcygeus muscle. |
|
Safety Profile |
Human poison by ingestion and subcutaneous routes. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. 1 198 PPRSOO PYROSULFURYL CHLORIDE Readdy absorbed through the skin. Human systemic effects by ingestion: convulsions, dyspnea, gastrointestinal effects. A severe skin and eye irritant. Incompatible with alkalies, NH3, antipyrine, phenol, iron and lead salts, iodine, KMn04. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a topical antibacterial agent, as an intermediate, hair dye component, and analytical reagent. |
|
Carcinogenicity |
Pyrogallol was not carcinogenic in mouse and rabbit chronic dermal studies. Mice were treated twice weekly with pyrogallol in acetone (50%) on the shaved flank for life. There was no increase in dermal or systemic tumors. A similar study in rabbits also revealed no skin tumors, although positive controls showed an increase in tumors in both mice and rabbits. Pyrogallol was considered to be cocarcinogenic when administered dermally three times a week together with the skin carcinogen benzo[a]pyrene for 440 days; pyrogallol administered alone caused no increase in skin tumors. |
InChI:InChI=1/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H
87-66-1 Relevant articles
Photocatalytic Degradation of 4,4′-Isopropylidenebis(2,6-dibromophenol) on Magnetite Catalysts vs. Ozonolysis Method: Process Efficiency and Toxicity Assessment of Disinfection By-Products
Balawejder, Maciej,Barylyak, Adriana,Bobitski, Yaroslav,Kisa?a, Joanna,Tomaszewska, Anna
, (2022/03/31)
Flame retardants have attracted growing ...
Study on in Vitro Preparation and Taste Properties of N-Ethyl-2-Pyrrolidinone-Substituted Flavan-3-Ols
Han, Zisheng,Ho, Chi-Tang,Jiang, Zongde,Lai, Guoping,Qin, Chunyin,Wan, Xiaochun,Wen, Mingchun,Zhai, Xiaoting,Zhang, Hui,Zhang, Liang
, (2022/04/07)
N-ethyl-2-pyrrolidinone-substituted flav...
RETRACTED ARTICLE: Selective photocatalytic conversion of guaiacol using g-C3N4 metal free nanosheets photocatalyst to add-value products
Rojas,Espinoza-Villalobos,Salazar,Escalona,Contreras,Melin,Laguna-Bercero,Sánchez-Arenillas,Vergara,Caceres-Jensen,Rodriguez-Becerra,Barrientos
, (2021/09/06)
Valorization of lignin into high valuabl...
Iron-catalyzed arene C-H hydroxylation
Cheng, Lu,Wang, Huihui,Cai, Hengrui,Zhang, Jie,Gong, Xu,Han, Wei
, p. 77 - 81 (2021/10/05)
The sustainable, undirected, and selecti...
87-66-1 Process route

-
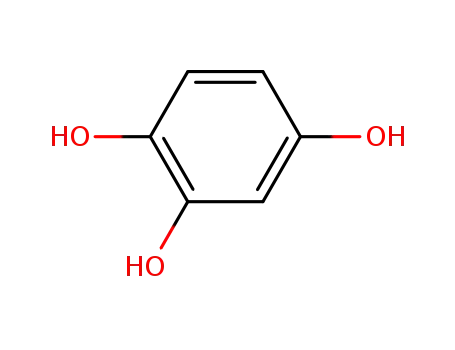
-
533-73-3
1,2,4-Trihydroxybenzene

-
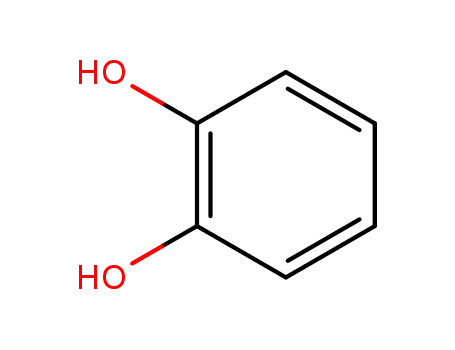
-
120-80-9,19481-10-8,37349-32-9
benzene-1,2-diol

-
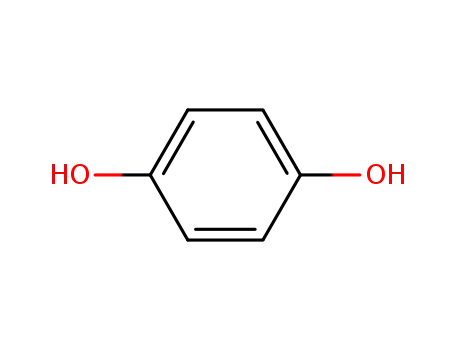
-
123-31-9,8027-02-9
hydroquinone

-
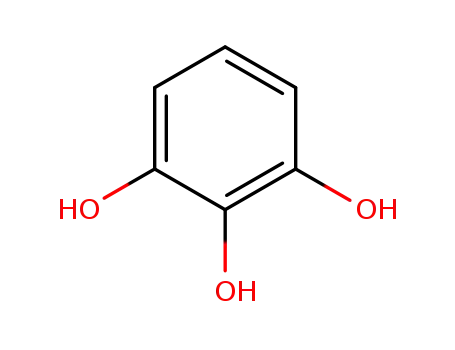
-
87-66-1
2-hydroxyresorcinol
| Conditions | Yield |
|---|---|
|
With
sodium chloride;
at 340 ℃;
for 1h;
Yield given;
|
|
|
With
sodium chloride;
at 340 ℃;
for 1h;
Yield given. Further byproducts given. Yields of byproduct given;
|
|
|
With
sodium chloride;
at 315 ℃;
for 2h;
Yield given. Further byproducts given. Yields of byproduct given;
|

-

-
533-73-3
1,2,4-Trihydroxybenzene

-
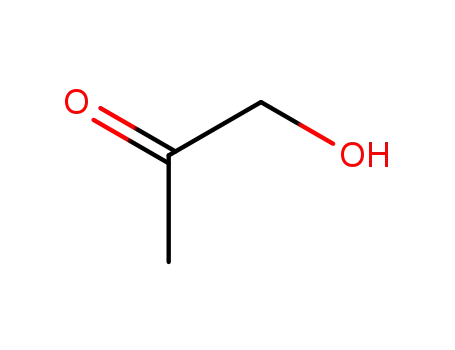
-
116-09-6
hydroxy-2-propanone

-
-
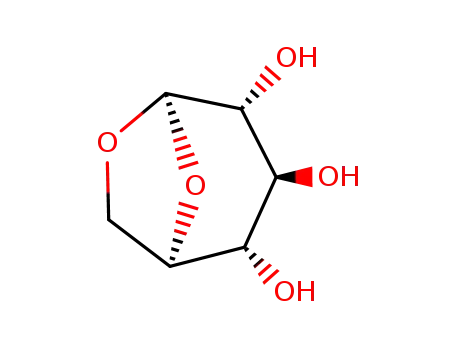
498-07-7
levoglucosan

-

-
87-66-1
2-hydroxyresorcinol
| Conditions | Yield |
|---|---|
|
With
sodium chloride;
at 340 ℃;
for 1h;
Yield given. Further byproducts given. Yields of byproduct given;
|
87-66-1 Upstream products
-
634-36-6
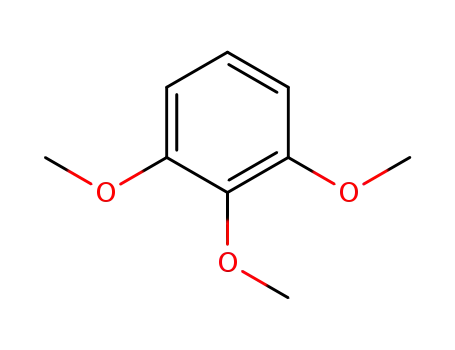
1,2,3-trimethoxybenzene
-
142-04-1
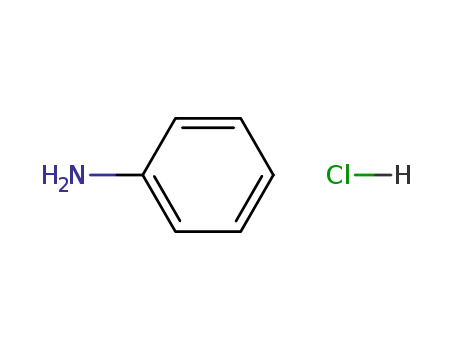
aniline hydrochloride
-
628-13-7
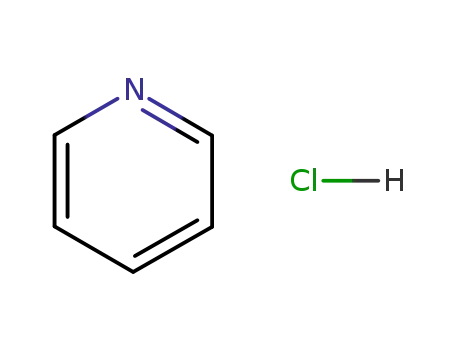
pyridine hydrochloride
-
149-91-7
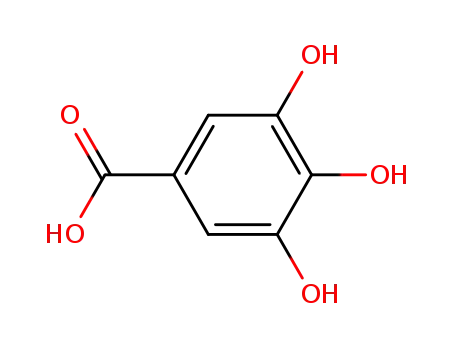
3,4,5-trihydroxybenzoic acid
87-66-1 Downstream products
-
41273-93-2
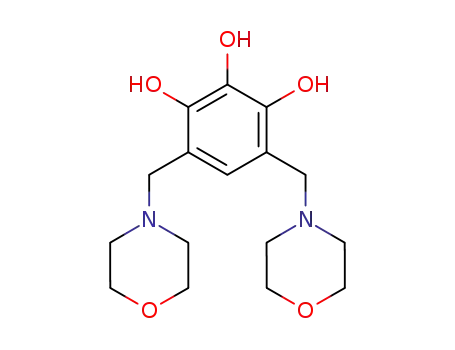
4,6-bis-morpholin-4-ylmethyl-benzene-1,2,3-triol
-
2107-77-9
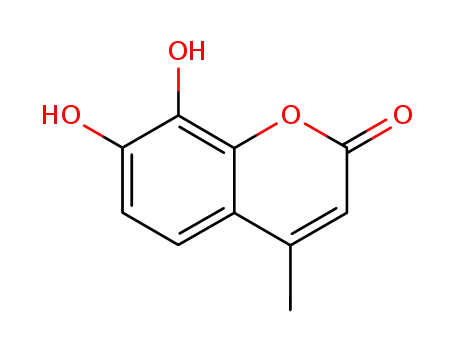
4-methyldaphnetin
-
3598-34-3
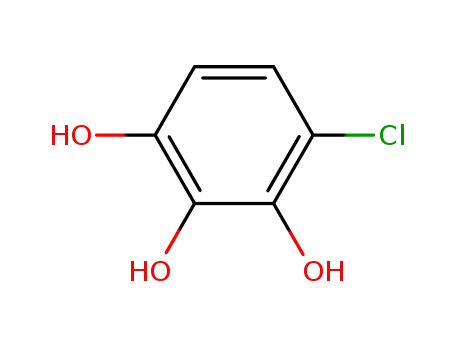
4-Chloro-1,2,3-trihydroxybenzene
-
87441-03-0
1,2,3-trihydroxy-4,6-dichlorobenzene
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
(S)-1-CBZ-3-PYRROLIDINOL
CAS:100858-32-0
-
Zolpidic acid
CAS:189005-44-5