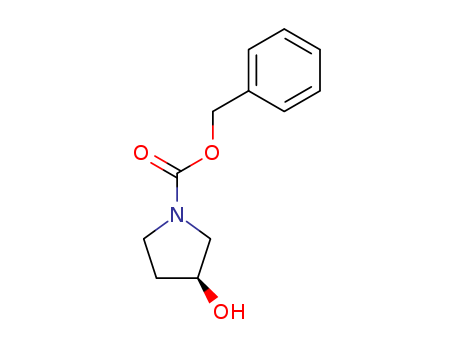
100858-32-0
- Product Name:(S)-1-CBZ-3-PYRROLIDINOL
- Molecular Formula:C12H15NO3
- Purity:99%
- Molecular Weight:221.256
Product Details
pd_meltingpoint:71-77 °C
Factory Sells Best Quality (S)-1-CBZ-3-PYRROLIDINOL 100858-32-0 with USP
- Molecular Formula:C12H15NO3
- Molecular Weight:221.256
- Vapor Pressure:3.75E-06mmHg at 25°C
- Melting Point:71-77 °C
- Refractive Index:1.589
- Boiling Point:370.7 °C at 760 mmHg
- PKA:14.72±0.20(Predicted)
- Flash Point:178 °C
- PSA:49.77000
- Density:1.263 g/cm3
- LogP:1.32770
(S)-1-CBZ-3-PYRROLIDINOL 95(Cas 100858-32-0) Usage
InChI:InChI=1/C12H15NO3/c14-11-6-7-13(8-11)12(15)16-9-10-4-2-1-3-5-10/h1-5,11,14H,6-9H2/t11-/m0/s1
100858-32-0 Relevant articles
Chiral Synthesis via Organoboranes. 6. Hydroboration. 74. Asymmetric Hydroboration of Representative Heterocyclic Olefins with Diisopinocamphenylborane. Synthesis of Heterocyclic Boronates and Heterocyclic Alcohols of Very High Enantiomeric Purity
Brown, Herbert C.,Prasad, J. V. N. Vara
, p. 2049 - 2054 (1986)
The hydroboration of representative hete...
Stereo-complementary bioreduction of saturated N-heterocyclic ketones
Li, Chao,Liu, Yan,Pei, Xiao-Qiong,Wu, Zhong-Liu
, p. 90 - 97 (2017)
The asymmetric bioreduction of several s...
Enantioselective reduction of heterocyclic ketones with low level of asymmetry using carrots
Machado, Naira Vieira,Omori, álvaro Takeo
, p. 475 - 480 (2021)
A whole spectrum of biocatalysts for asy...
Regio- and stereoselective biohydroxylations with a recombinant escherichia coli expressing P450pyr monooxygenase of sphingomonas Sp. HXN-200
Zhang, Wei,Tang, Weng Lin,Wang, Zunsheng,Li, Zhi
, p. 3380 - 3390 (2010)
A recombinant Escherichia coli expressin...
Ultrafast chiral separations for high throughput enantiopurity analysis
Barhate, Chandan L.,Joyce, Leo A.,Makarov, Alexey A.,Zawatzky, Kerstin,Bernardoni, Frank,Schafer, Wes A.,Armstrong, Daniel W.,Welch, Christopher J.,Regalado, Erik L.
supporting information, p. 509 - 512 (2017/01/13)
Recent developments in fast chromatograp...
BRUTON'S TYROSINE KINASE INHIBITORS
-
Page/Page column 81; 82, (2014/05/24)
Disclosed herein are compounds that form...
100858-32-0 Process route
-
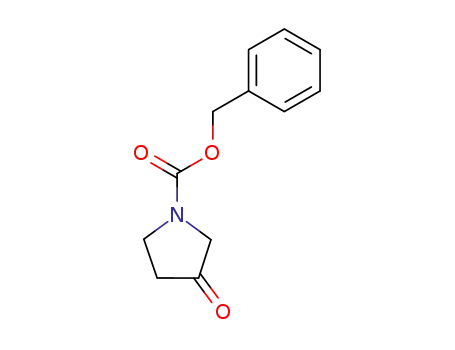
-
130312-02-6
benzyl 3-oxopyrrolidine-1-carboxylate

-
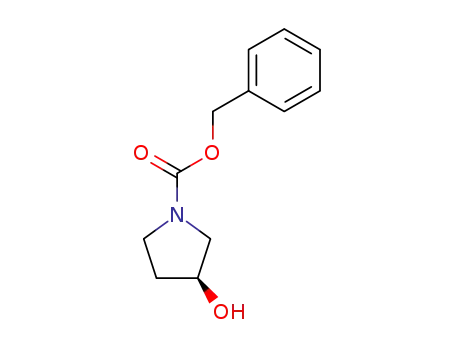
-
100858-32-0
(S)-3-hydroxy-1-pyrrolidinecarboxylic acid phenylmethyl ester
| Conditions | Yield |
|---|---|
|
With
recombinant Rhodococcus erythropolis DSM 43297 ketoredutase; nicotinamide adenine dinucleotide;
In
aq. phosphate buffer; isopropyl alcohol;
at 50 ℃;
for 21h;
pH=7.0;
stereoselective reaction;
Enzymatic reaction;
|
86% |
|
With
water;
at 20 ℃;
for 72h;
enantioselective reaction;
|
41% |
-
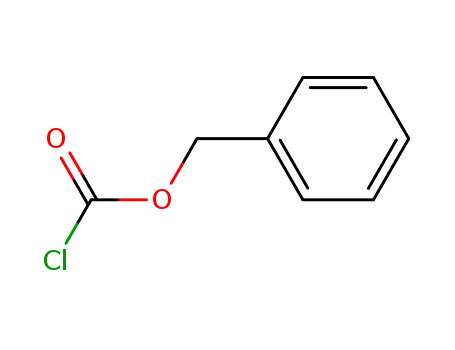
-
501-53-1,94274-21-2
benzyl chloroformate

-
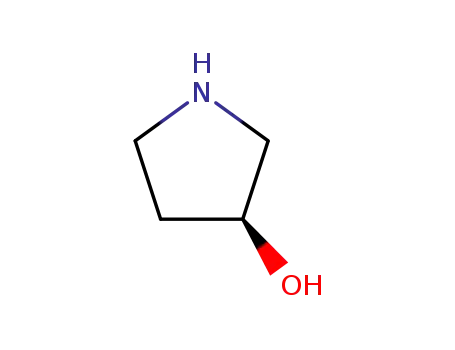
-
100243-39-8
3-hydroxypyrrolidine

-

-
100858-32-0
(S)-3-hydroxy-1-pyrrolidinecarboxylic acid phenylmethyl ester
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
dichloromethane;
at 5 - 20 ℃;
for 48h;
|
92% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
100858-32-0 Upstream products
-
31970-04-4
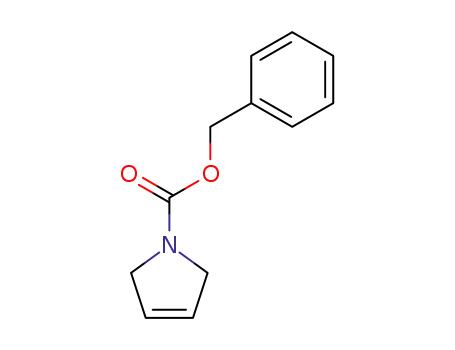
1-carboxybenzyl-3-pyrrolidine
-
501-53-1

benzyl chloroformate
-
122536-94-1
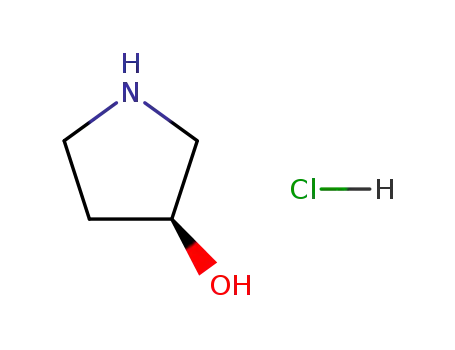
(S)-3-hydroxypyrrolidine hydrochloride
-
100243-39-8

3-hydroxypyrrolidine
100858-32-0 Downstream products
-
122536-69-0
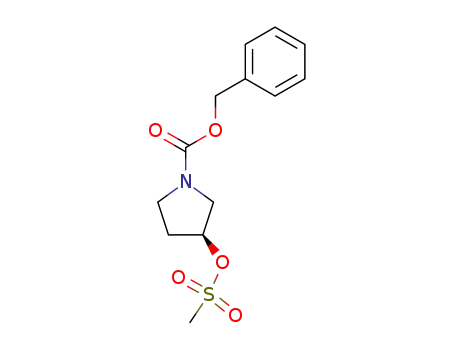
(S)-3-<(methylsulfonyl)oxy>-1-pyrrolidinecarboxylic acid phenylmethyl ester
-
158654-83-2
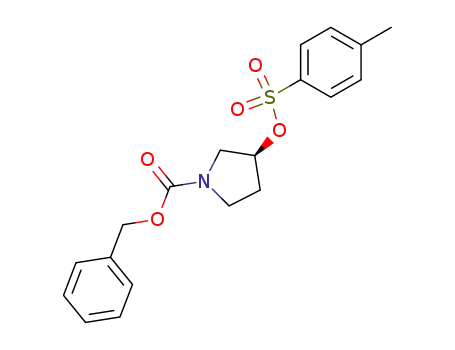
benzyl (3S)-3-(p-tolylsulfonyloxy)pyrrolidine-1-carboxylate
-
328404-62-2
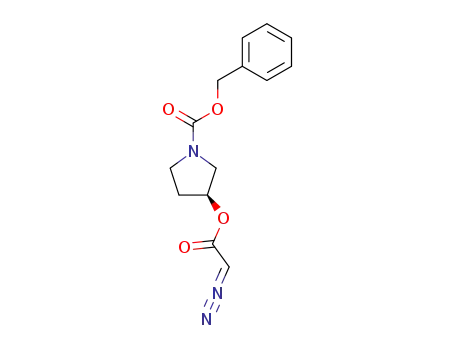
(S)-3-[(1-benzyloxy-carbonyl)-pyrrolidinyl]-α-diazoacetate
-
163457-21-4
(R)-benzyl 3-fluoropyrrolidine-1-carboxylate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
4-Bromo-2-fluorobiphenyl
CAS:41604-19-7
-
Pyrogallic Acid
CAS:87-66-1