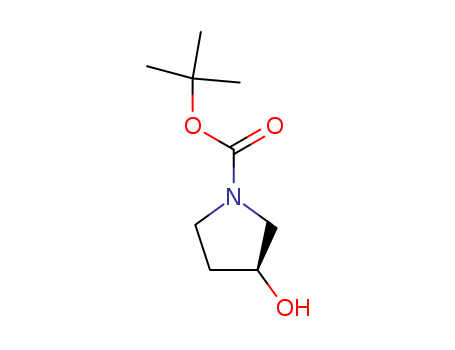
101469-92-5
- Product Name:N-(tert-Butoxycarbonyl)-(S)-(+)-3-pyrrolidinol
- Molecular Formula:C9H17NO3
- Purity:99%
- Molecular Weight:187.239
Product Details
pd_meltingpoint:60-64 °C
Appearance:white to light yellow crystal powder
Reliable factory customized supply N-(tert-Butoxycarbonyl)-(S)-(+)-3-pyrrolidinol 101469-92-5
- Molecular Formula:C9H17NO3
- Molecular Weight:187.239
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.000742mmHg at 25°C
- Melting Point:60-64 °C
- Refractive Index:27 ° (C=1, MeOH)
- Boiling Point:273.3 °C at 760 mmHg
- PKA:14.74±0.20(Predicted)
- Flash Point:119.1 °C
- PSA:49.77000
- Density:1.142 g/cm3
- LogP:0.92600
N-(tert-Butoxycarbonyl)-(S)-(+)-3-pyrrolidinol(Cas 101469-92-5) Usage
InChI:InChI=1/C9H17NO3/c1-9(2,3)13-8(12)10-5-4-7(11)6-10/h7,11H,4-6H2,1-3H3/t7-/m1/s1
101469-92-5 Relevant articles
Synthesis of piperidinyl and pyrrolidinyl butyrates for potential in vivo measurement of cerebral butyrylcholinesterase activity
Kikuchi, Tatsuya,Fukushi, Kiyoshi,Ikota, Nobuo,Ueda, Takao,Nagatsuka, Shin-Ichiro,Arano, Yasushi,Irie, Toshiaki
, p. 31 - 41 (2001)
Biochemical changes in postmortem brains...
Selection of microbial biocatalysts for the reduction of cyclic and heterocyclic ketones
Bianchi, Paola,Varela, Romina Fernández,Bianchi, Dario A.,Kemppainen, Minna,Iribarren, Adolfo M.,Lewkowicz, Elizabeth
, p. 137 - 144 (2017)
The reduction of carbonyl compounds play...
Enantioselective reduction of heterocyclic ketones with low level of asymmetry using carrots
Machado, Naira Vieira,Omori, álvaro Takeo
, p. 475 - 480 (2021/09/27)
A whole spectrum of biocatalysts for asy...
NOVEL OXADIAZOLES
-
Page/Page column 62-63, (2020/05/15)
The present invention relates to novel c...
PDE9 inhibitor and application thereof
-
Paragraph 0256-0258, (2019/04/17)
The invention belongs to the technical f...
SUBSTITUTED 2-HYDROGEN-PYRAZOLE DERIVATIVE SERVING AS ANTICANCER DRUG
-
Paragraph 0061; 0172; 0173, (2018/02/04)
Disclosed is a substituted 2H-pyrazole d...
101469-92-5 Process route
-
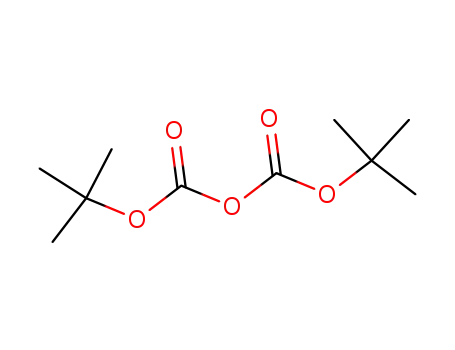
-
24424-99-5
di-tert-butyl dicarbonate

-
-
100243-39-8
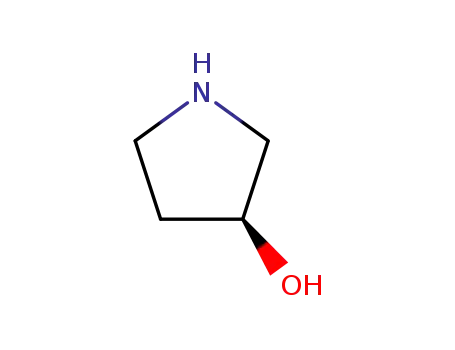
3-hydroxypyrrolidine

-
-
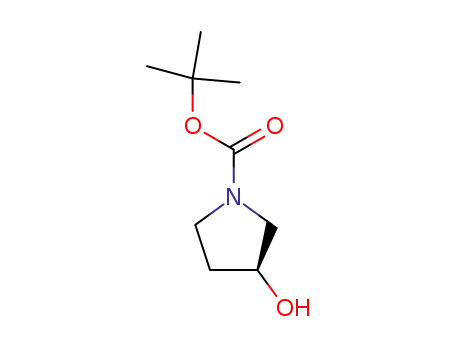
101469-92-5,83220-73-9
(S)-3-hydroxy-pyrrolidine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
tetrahydrofuran;
at 20 ℃;
for 5h;
|
100% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
100% |
|
With
triethylamine;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 48h;
|
97.2% |
|
With
triethylamine;
In
tetrahydrofuran;
at 0 ℃;
for 4h;
|
94% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
at 20 ℃;
|
92% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
92% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 3h;
|
87% |
|
With
potassium carbonate;
In
1,4-dioxane;
Yield given;
|
|
|
With
triethylamine;
In
methanol;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
In
chloroform;
at 20 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
at 25 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
at 18 - 25 ℃;
for 1h;
|
|
|
With
triethylamine;
In
methanol; water;
at 25 ℃;
for 2h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
3-hydroxypyrrolidine;
With
methanol;
In
water;
at 20 ℃;
for 1h;
di-tert-butyl dicarbonate;
With
sodium hydroxide;
In
water;
at 20 ℃;
for 18h;
|
398 mg |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 18h;
Inert atmosphere;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
In
tetrahydrofuran;
at 20 ℃;
|
-

-
24424-99-5
di-tert-butyl dicarbonate

-
-
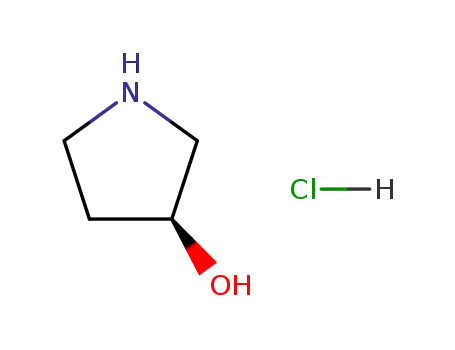
122536-94-1,111810-68-5
(S)-3-hydroxypyrrolidine hydrochloride

-

-
101469-92-5,83220-73-9
(S)-3-hydroxy-pyrrolidine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
methanol; dichloromethane;
at 0 ℃;
for 1h;
|
96% |
|
With
triethylamine;
In
methanol;
at 0 - 20 ℃;
for 6h;
|
95% |
|
With
triethylamine;
In
methanol;
at 20 ℃;
for 6h;
|
95% |
|
With
sodium hydrogencarbonate;
In
water;
at 25 - 30 ℃;
for 10h;
|
95% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 12h;
|
77.3% |
|
With
triethylamine;
In
methanol;
at 20 ℃;
Cooling with ice;
|
1.9 g |
101469-92-5 Upstream products
-
24424-99-5

di-tert-butyl dicarbonate
-
101469-91-4
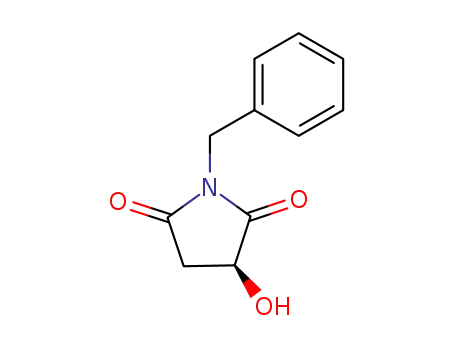
(3S)-1-benzyl-3-hydroxypyrrolidine-2,5-dione
-
100243-39-8

3-hydroxypyrrolidine
-
101385-90-4
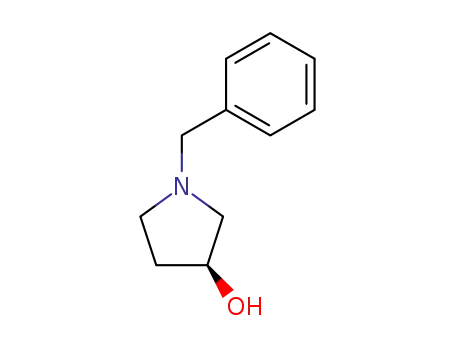
(S)-N-benzyl-3-hydroxypyrrolidine
101469-92-5 Downstream products
-
132945-75-6
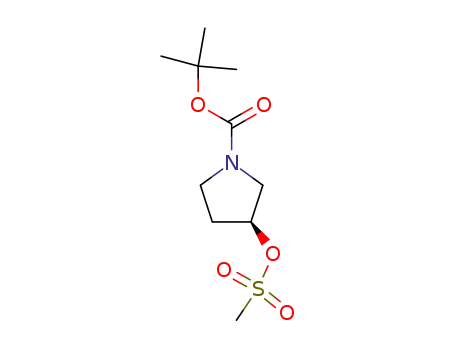
(S)-tert-butyl 3-(methylsulfonyloxy)pyrrolidine-1-carboxylate
-
847942-82-9
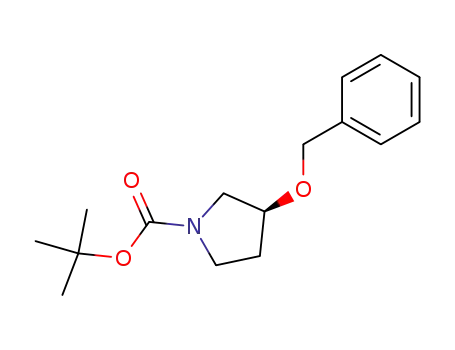
(3S)-N-(tert-butyloxycarbonyl)-3-(benzyloxy)pyrrolidine
-
330797-85-8
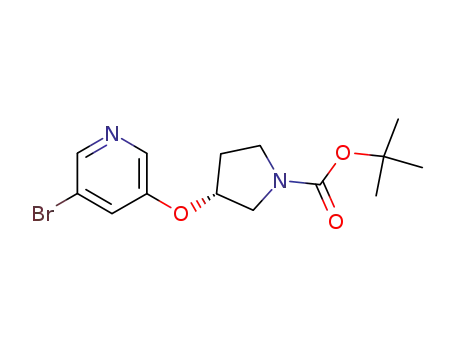
(R)-3-(5-bromo-pyridin-3-yloxy)-pyrrolidine-1-carboxylic acid tert-butyl ester
-
150610-40-5
tert-butyl (3R)-3-[4-(2-ethoxy-2-oxoacetyl)phenoxy]-1-pyrrolidinecarboxylate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
IMAZALIL
CAS:35554-44-0
-
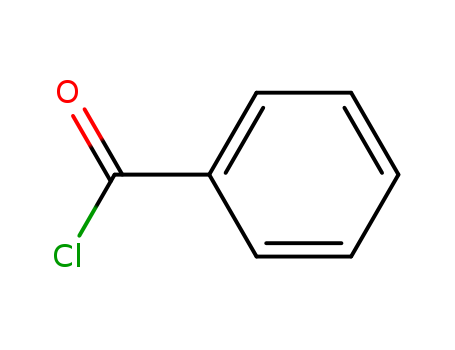
Benzoyl chloride
CAS:98-88-4