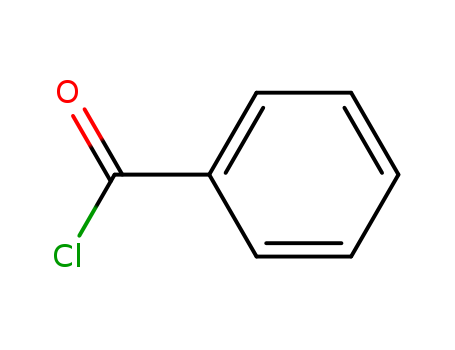
98-88-4
- Product Name:Benzoyl chloride
- Molecular Formula:C7H5ClO
- Purity:99%
- Molecular Weight:140.569
Product Details
pd_meltingpoint:-1 ºC
Appearance:Colorless liquid
Benzoyl chloride Good Supplier In Bulk Supply High Purity 98-88-4
- Molecular Formula:C7H5ClO
- Molecular Weight:140.569
- Appearance/Colour:Colorless liquid
- Vapor Pressure:1 mm Hg ( 32 °C)
- Melting Point:-1 ºC
- Refractive Index:1.553
- Boiling Point:197.2 ºC at 760 mmHg
- Flash Point:70.7 ºC
- PSA:17.07000
- Density:1.213 g/cm3
- LogP:2.06560
Benzoyl chloride(Cas 98-88-4) Usage
|
Chemical Description |
Benzoyl chloride is an organic compound used as a reagent in organic synthesis. |
|
Physical and Chemical Properties |
Its pure product is a colorless and transparent flammable liquid, which is smoking exposed to air in the air. In Industry, it is slightly pale yellow, with a strong pungent odor. Its steam has a strong stimulating effect for eye mucous membranes, skin and respiratory tract, by stimulating the mucous membranes and eyes tear. Benzoyl chloride Melting point is-1.0 ℃, boiling point is 197.2 ℃, and the relative density is 1.212 (20 ℃), while a flash point is 72 ℃, and refractive index (n20) is 1.554. It is soluble in the ether, chloroform, benzene and carbon disulfide. It can gradually decomposed in water or ethanol, ammonia, which generates benzoic acid, generating benzamide, ethyl benzoate and hydrogen chloride. In the laboratory, it can be obtained by distillation of benzoic acid and phosphorus pentachloride under anhydrous conditions. Industrial production process can be obtained by the use of thionyl chloride benzaldehyde. Benzoyl chloride is an important intermediate for preparing dyes, perfumes, organic peroxides, resins and drugs. It is also used in photography and artificial tannin production, which was formerly used as an irritant gas in chemical warfare. |
|
Application |
Benzoyl chloride is used for organic synthesis, dye and pharmaceutical raw material, manufacturing initiator benzoyl peroxide, t-butyl peroxybenzoate, pesticides and herbicides. In pesticides, it is a new insecticide, which is inducible isoxazole parathion (Isoxathion, Karphos) intermediate. Benzoyl chloride is an important benzoyl and benzyl reagent. Most of benzoyl chloride is used in the production of benzoyl peroxide, and secondly for the production of benzophenone, benzyl benzoate, benzyl cellulose. Benzoyl peroxide catalyzes polymerization initiator for the monomer plastic, polyester, epoxy, acrylic resin production, self-curing agent, which is a glass fiber material, fluorine rubber, silicone crosslinking agents, oil refined, bleached flour, fiber decolorizing. |
|
Production Methods |
Benzoyl chloride can be prepared from benzoic acid by reaction with PCl5 or SOCl2, from benzaldehyde by treatment with POCl3 or SO2 Cl2, from benzotrichloride by partial hydrolysis in the presence of H2SO4 or FeCl3, from benzal chloride by treatment with oxygen in a radical source, and from several other miscellaneous reactions. Benzoyl chloride can be reduced to benzaldehyde, oxidized to benzoyl peroxide, chlorinated to chlorobenzoyl chloride and sulfonated to m-sulfobenzoic acid. It will undergo various reactions with organic reagents. For example, it will add across an unsaturated (alkene or alkyne) bond in the presence of a catalyst to give the phenylchloroketone: |
|
Definition |
ChEBI: Benzoyl chloride is an acyl chloride consisting of benzene in which a hydrogen is replaced by an acyl chloride group. It is an important chemical intermediate for the manufacture of other chemicals, dyes, perfumes, herbicides and pharmaceuticals. It has a role as a carcinogenic agent. It is an acyl chloride and a member of benzenes. It is functionally related to a benzoic acid.? |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 73, p. 702, 1951 DOI: 10.1021/ja01146a061Synthesis, p. 306, 1983 DOI: 10.1055/s-1983-30314 |
|
General Description |
Benzoyl chloride appears as a colorless fuming liquid with a pungent odor. Flash point 162 °F. Lachrymator, irritating to skin and eyes. Corrosive to metals and tissue. Density 10.2 lb / gal. Used in medicine and in the manufacture of other chemicals. |
|
Reactivity Profile |
Benzoyl chloride reacts violently with protic solvents such as alcohols, with amines and amides (for example dimethylformamide [Bretherick 1979 p. 6] ) and with inorganic bases. Causes the violent decomposition of dimethyl sulfoxide [Chem. Eng. News 35(9): 87 1957]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Friedel-Crafts acylation of naphthalene using Benzoyl chloride, catalyzed by AlCl3, must be conducted above the melting point of the mixture, or the reaction may be violent [Clar, E. et al., Tetrahedron, 1974, 30, 3296]. |
|
Hazard |
Highly toxic. Strong irritant to skin, eyes, and mucous membranes, and via ingestion, inhala- tion. Upper respiratory tract irritant. Probable car- cinogen. |
|
Health Hazard |
INHALATION: may irritate eyes, nose and throat. INGESTION: causes acute discomfort. SKIN: causes irritation and burning. |
|
Chemical Reactivity |
Reactivity with Water Slow reaction with water to produce hydrochloric acid fumes. The reaction is more rapid with steam; Reactivity with Common Materials: Slow corrosion of metals but no immediate danger; Stability During Transport: Not pertinent; Neutralizing Agents for Acids and Caustics: Soda ash and water, lime; Polymerization: Does not occur; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile |
Confirmed carcinogen withexperimental tumorigenic data by skin contact. Humansystemic effects by inhalation: unspecified effects onolfaction and respiratory systems. Corrosive effects on theskin, eyes, and mucous membranes by inhalation.Flammable whe |
|
Potential Exposure |
Benzoyl chloride is used as a chemical intermediate; in organic synthesis; to produce other chemicals, dyes, perfumes, herbicides, and medicines. |
|
Shipping |
UN 1736 Benzoylchloride, Hazard class: 8; Labels: 8—Corrosive material. |
|
Purification Methods |
A solution of benzoyl chloride (300mL) in *C6H6 (200mL) is washed with two 100mL portions of cold 5% NaHCO3 solution, separated, dried with CaCl2 and distilled [Oakwood & Weisgerber Org Synth III 113 1955]. Repeated fractional distillation at 4mm Hg through a glass helices-packed column (avoiding porous porcelain or silicon-carbide boiling chips, and hydrocarbon or silicon greases on the ground joints) gave benzoyl chloride that did not darken on addition of AlCl3. Further purification is achieved by adding 3 mole% each of AlCl3 and toluene, standing overnight, and distilling off the benzoyl chloride at 1-2mm [Brown & Jenzen J Am Chem Soc 80 2291 1958]. Refluxing for 2hours with an equal weight of thionyl chloride before distillation has also been used. [Beilstein 9 IV 721.] Strong IRRITANT. Use in a fume cupboard. |
|
Incompatibilities |
May form explosive mixture with air. Contact with heat, hot surfaces, and flames causes decomposition, forming phosgene and hydrogen chloride. Water contact may be violent; forms hydrochloric acid. Reactions with amines, alcohols, alkali metals, dimethyl sulfoxide, strong oxidizers, and metal salts may be violent, causing fire and explosions. Attacks metals in the presence of moisture, forming explosive hydrogen gas. Attacks some plastics, rubber or coatings. |
|
Waste Disposal |
Pour into sodium bicarbonate solution and flush to sewer. |
InChI:InChI=1/C7H5ClO/c8-7(9)6-4-2-1-3-5-6/h1-5H
98-88-4 Relevant articles
One-step Conversion of Amides and Esters to Acid Chlorides with PCl3
Li, Fangshao,Wu, Xiaofang,Guo, Fengzhe,Tang, Zi-Long,Xiao, Jing
supporting information, p. 4314 - 4317 (2021/07/16)
A general and efficient iodine-promoted ...
Photochemical Activation of Aromatic Aldehydes: Synthesis of Amides, Hydroxamic Acids and Esters
Nikitas, Nikolaos F.,Apostolopoulou, Mary K.,Skolia, Elpida,Tsoukaki, Anna,Kokotos, Christoforos G.
supporting information, p. 7915 - 7922 (2021/05/03)
A cheap, facile and metal-free photochem...
Remarkably Efficient Iridium Catalysts for Directed C(sp2)-H and C(sp3)-H Borylation of Diverse Classes of Substrates
Chattopadhyay, Buddhadeb,Hassan, Mirja Md Mahamudul,Hoque, Md Emdadul
supporting information, p. 5022 - 5037 (2021/05/04)
Here we describe the discovery of a new ...
Immobilization of (l)-valine and (l)-valinol on SBA-15 nanoporous silica and their application as chiral heterogeneous ligands in the Cu-catalyzed asymmetric allylic oxidation of alkenes
Samadi, Saadi,Ashouri, Akram,Rashid, Hersh I,Majidian, Shiva,Mahramasrar, Mahsa
supporting information, p. 17630 - 17641 (2021/10/04)
SBA-15 nanoporous silica was synthesized...
98-88-4 Process route
-
-
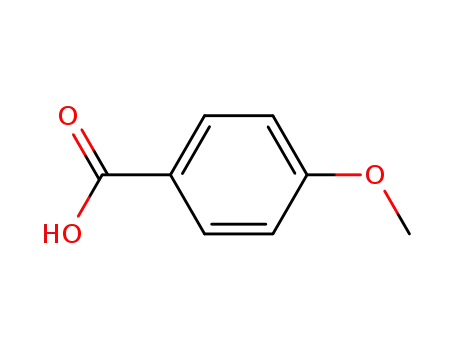
100-09-4
4-methoxybenzoic acid

-
-
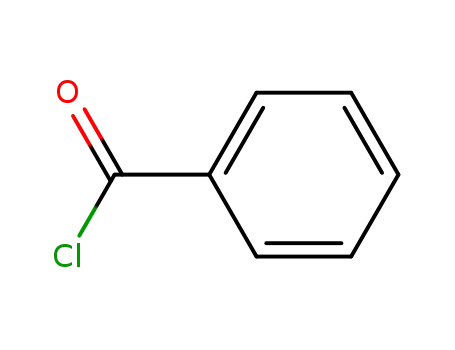
98-88-4
benzoyl chloride

-
-
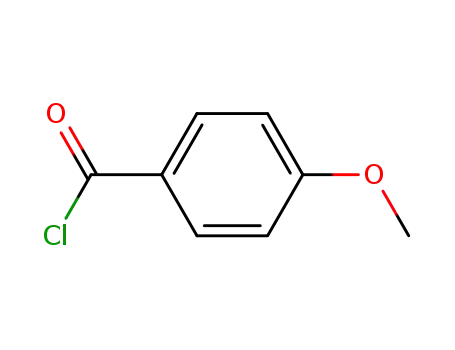
100-07-2
4-methoxy-benzoyl chloride
| Conditions | Yield |
|---|---|
|
With
Benzotrichlorid;
iron(III) chloride;
In
benzene;
at 55 ℃;
Product distribution;
Rate constant;
Thermodynamic data;
ΔE(excit.), var. temp.;
|
-
-
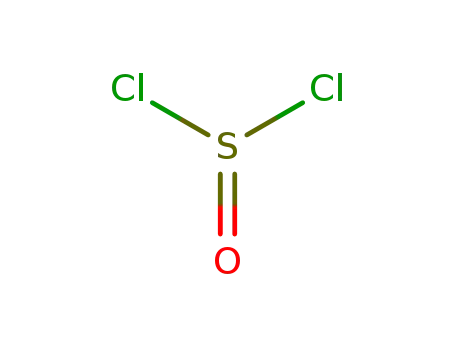
7719-09-7
thionyl chloride

-
-
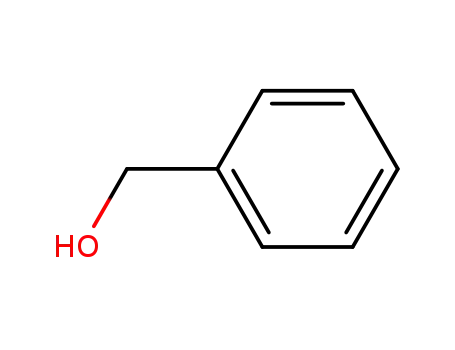
100-51-6,185532-71-2
benzyl alcohol

-
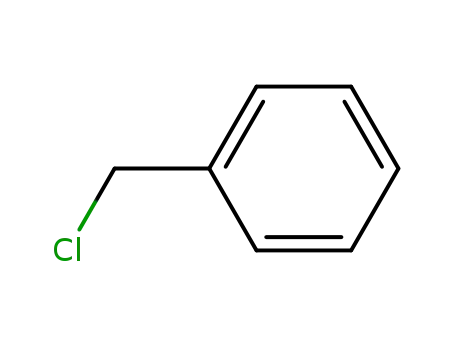
-
100-44-7
benzyl chloride

-

-
98-88-4
benzoyl chloride
| Conditions | Yield |
|---|---|
|
at 180 ℃;
|
98-88-4 Upstream products
-
110-86-1
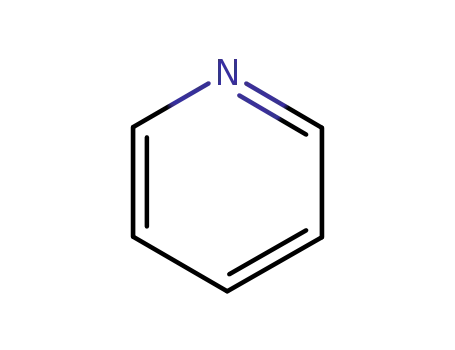
pyridine
-
98-59-9
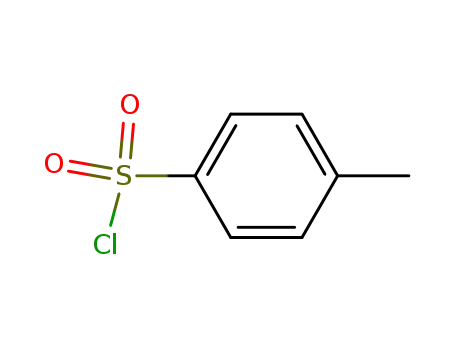
p-toluenesulfonyl chloride
-
65-85-0
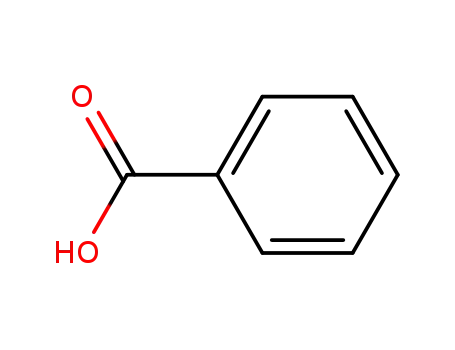
benzoic acid
-
93-58-3
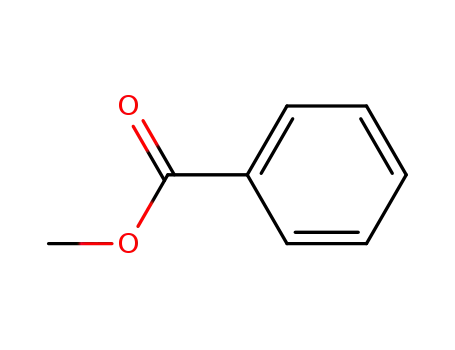
benzoic acid methyl ester
98-88-4 Downstream products
-
32849-03-9
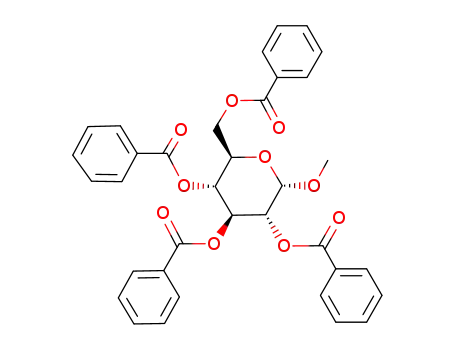
methyl 2,3,4,6-tetra-O-benzoyl-α-D-glycopyranoside
-
6638-76-2
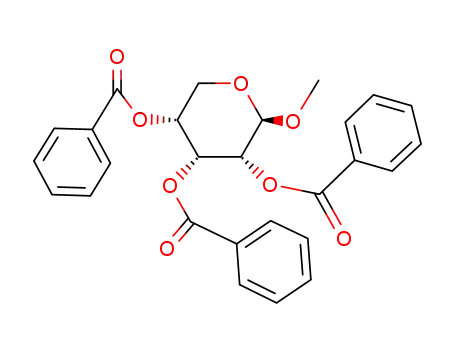
methyl 2,3,4-tri-O-benzoyl-β-D-ribopyranoside
-
121143-61-1
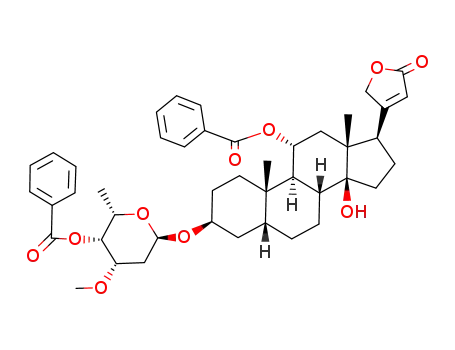
3β-(O4-benzoyl-O3-methyl-α-L-lyxo-2,6-dideoxy-hexopyranosyloxy)-11α-benzoyloxy-14-hydroxy-5β,14β-card-20(22)-enolide
-
108021-00-7
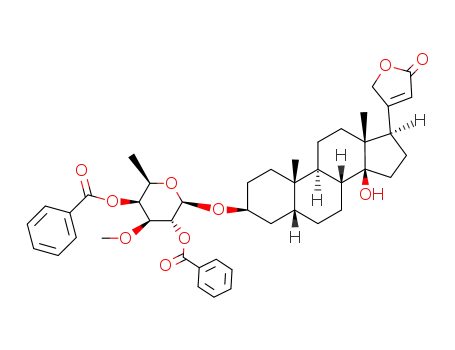
3β-(O2,O4-dibenzoyl-O3-methyl-β-D-fucopyranosyloxy)-14-hydroxy-5β,14β-card-20(22)-enolide
Relevant Products
-
N-(tert-Butoxycarbonyl)-(S)-(+)-3-pyrrolidinol
CAS:101469-92-5
-
Gallic acid
CAS:5995-86-8