35554-44-0
- Product Name:IMAZALIL
- Molecular Formula:C14H14Cl2N2O
- Purity:99%
- Molecular Weight:297.184
Product Details
pd_meltingpoint:52.7 °C
Appearance:slightly yellow to brown solidified oil
Chinese Factory Supply Wholesale IMAZALIL 35554-44-0 with Cheap Price
- Molecular Formula:C14H14Cl2N2O
- Molecular Weight:297.184
- Appearance/Colour:slightly yellow to brown solidified oil
- Vapor Pressure:1.58 x l0-4 Pa (20 °C)
- Melting Point:52.7 °C
- Refractive Index:1.5680 (estimate)
- Boiling Point:448.5 °C at 760 mmHg
- PKA:6.53 (weak base)
- Flash Point:225.1 °C
- PSA:27.05000
- Density:1.23 g/cm3
- LogP:4.13380
Imazalil(Cas 35554-44-0) Usage
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
Imazalil is an imidazole derivative. |
|
Safety Profile |
Poison by ingestion and intraperitoneal routes. Experimental reproductive effects. A skin and eye irritant. When heated to decomposition it emits toxic fumes of Cland NOx. |
|
Veterinary Drugs and Treatments |
Although no dosage forms are currently commercially available for topical use in the USA, Enilconazole is used topically for treating dermatophytosis in small animals and horses using compounded products. A commercially available topical rinse Imaverol? (Janssen) 10% is available with canine, bovine and equine use labeling in many countries. Intranasal instillation of enilconazole after plaque debridement has also been shown useful in treating nasal aspergillosis in small animals. Use of topical enilconazole on cats with dermatophytosis is somewhat controversial as there are apparently no products with feline labeling available in Europe or Canada. There are preliminary reports of safely and successfully using enilconazole on dermatophytic cats in combination with oral itraconazole. A topical product and a poultry environmental disinfectant product (Clinafarm EC?) is available in the USA. It is technically illegal to use this product other than it is labeled; it is an EPA licensed product in the USA. |
|
Metabolic pathway |
Published information is available on the metabolism of imazalil in plants and soils. The principal metabolite in plants and soils is 1-[2-(2,4- dichlorophenyl)-2-hydroxyethyl]-1H-imidazole. |
|
Degradation |
Imazalil is very stable to hydrolysis in dilute acids and alkalis at room temperature, in the absence of light. It is also stable to light under normal storage conditions. |
|
General Description |
Slightly yellow to brown solidified oil. Non-corrosive. Used as a fungicide. |
InChI:InChI=1/C14H14Cl2N2O/c1-2-7-19-14(9-18-6-5-17-10-18)12-4-3-11(15)8-13(12)16/h2-6,8,10,14H,1,7,9H2
35554-44-0 Relevant articles
O-allylation method of alpha,beta-diaryl substituted ethanol
-
Paragraph 0039-0043, (2020/03/09)
The invention belongs to the technical f...
Enilconazole bulk drug preparation method
-
Paragraph 0039-0055, (2019/11/20)
The invention discloses an enilconazole ...
Enilconazole preparation method
-
Paragraph 0023-0034, (2018/07/30)
The invention discloses an enilconazole ...
Design and synthesis of novel imidazole derivatives as potent inhibitors of allene oxide synthase(CYP74)
Oh, Keimei,Murofushi, Noboru
, p. 3707 - 3711 (2007/10/03)
Allene oxide synthase (AOS) is a key enz...
35554-44-0 Process route
-
-
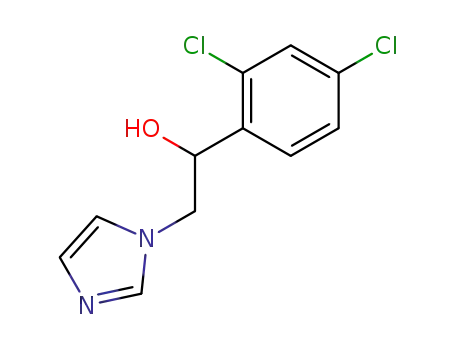
24155-42-8
1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol

-
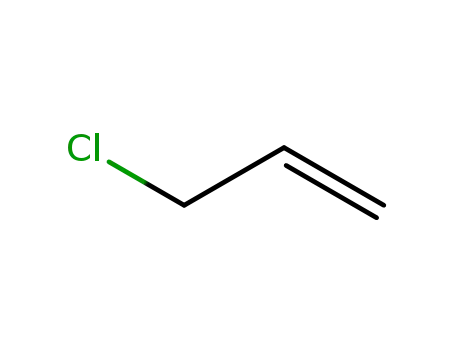
-
107-05-1
3-chloroprop-1-ene

-
![1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)ethyl]-1H-imidazole](/upload/2024/12/951806a2-8d6c-4029-b878-892441b72aea.png)
-
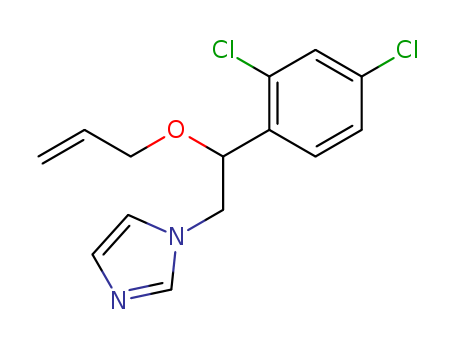
35554-44-0
1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)ethyl]-1H-imidazole
| Conditions | Yield |
|---|---|
|
1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol;
With
potassium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 1h;
3-chloroprop-1-ene;
In
water; dimethyl sulfoxide;
at 30 ℃;
for 3h;
Solvent;
Temperature;
|
95.6% |
|
1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol;
With
potassium hydroxide;
In
water; dimethyl sulfoxide;
at 20 - 35 ℃;
for 1h;
3-chloroprop-1-ene;
In
water; dimethyl sulfoxide;
at 35 - 40 ℃;
Reagent/catalyst;
|
68% |
-
-
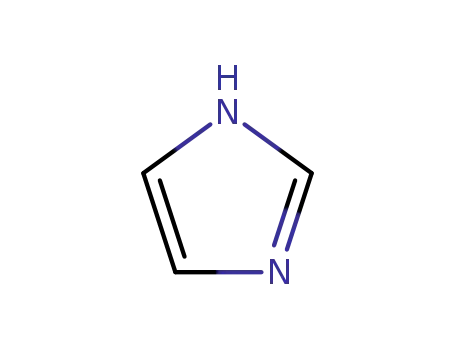
288-32-4
1H-imidazole

-
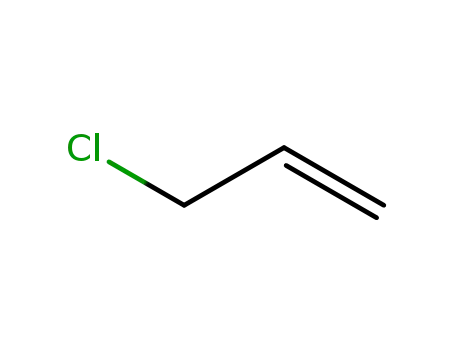
-
107-05-1
3-chloroprop-1-ene

-
-
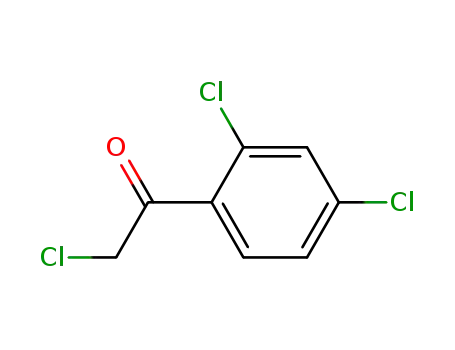
4252-78-2
2,2',4'-trichloroacetophenone

-
![1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)ethyl]-1H-imidazole](/upload/2024/12/951806a2-8d6c-4029-b878-892441b72aea.png)
-
35554-44-0
1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)ethyl]-1H-imidazole
| Conditions | Yield |
|---|---|
|
2,2',4'-trichloroacetophenone;
With
tris(triphenylphosphine)ruthenium(II) chloride; acetic acid; triethylamine;
at 70 ℃;
for 9h;
1H-imidazole;
With
N,N-dimethyl acetamide; sodium hydroxide;
at 50 - 100 ℃;
for 5h;
3-chloroprop-1-ene;
at 105 ℃;
for 6h;
Reagent/catalyst;
Solvent;
|
64.4% |
35554-44-0 Upstream products
-
106-95-6
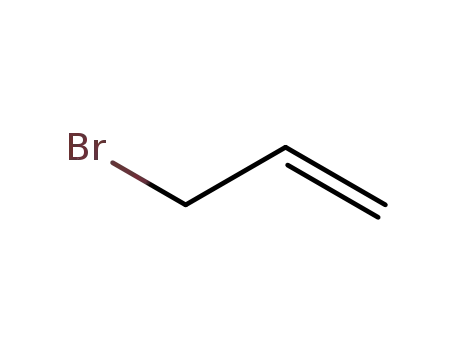
allyl bromide
-
24155-42-8

1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethan-1-ol
-
288-32-4

1H-imidazole
-
107-05-1
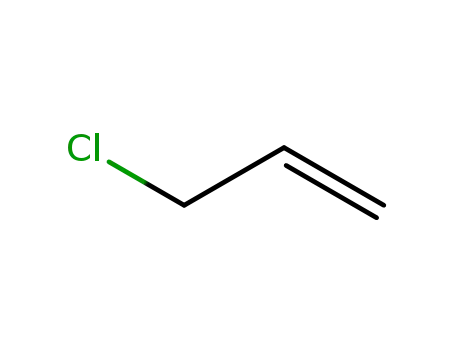
3-chloroprop-1-ene
35554-44-0 Downstream products
-
57265-11-9
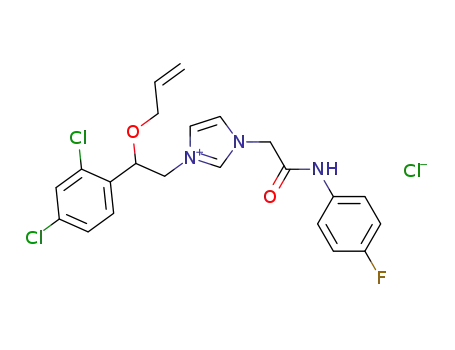
1-[β-(Allyloxy)-2,4-dichlorophenethyl]-3-[N-(p-fluorophenyl)-carbamoylmethyl]imidazolium chloride
-
166734-82-3
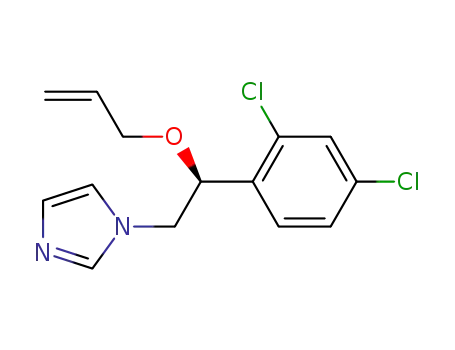
S-(+)-imazalil
-
166734-81-2
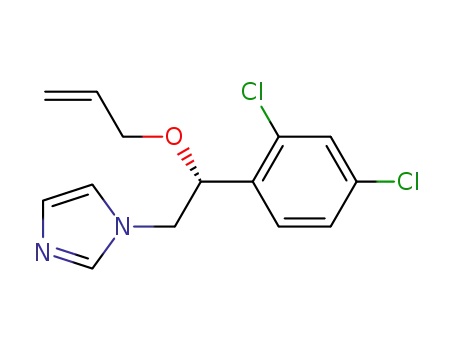
R-(?)-imazalil
Relevant Products
-
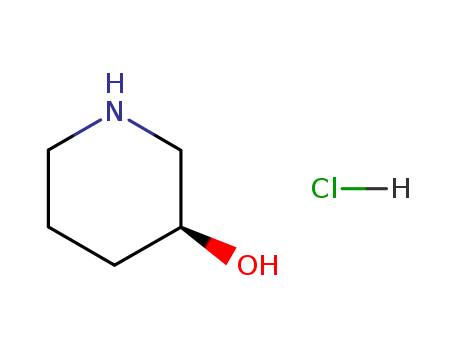
(S)-3-Hydroxypiperidine hydrochloride
CAS:475058-41-4
-
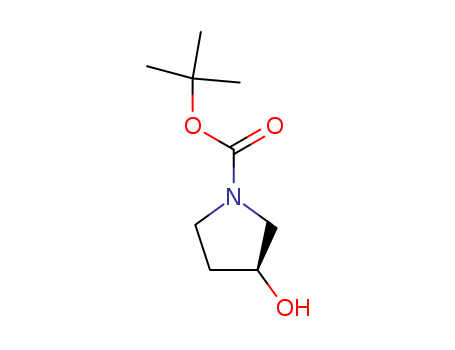
N-(tert-Butoxycarbonyl)-(S)-(+)-3-pyrrolidinol
CAS:101469-92-5