52927-22-7
- Product Name:6-Cyano-2-naphthol
- Molecular Formula:C11H7NO
- Purity:99%
- Molecular Weight:169.183
Product Details
pd_meltingpoint:165.5-170.5 °C(lit.)
Appearance:brown crystalline solid
Manufacturer supply high quality 6-Cyano-2-naphthol 52927-22-7 with ISO standards
- Molecular Formula:C11H7NO
- Molecular Weight:169.183
- Appearance/Colour:brown crystalline solid
- Vapor Pressure:2.04E-06mmHg at 25°C
- Melting Point:165.5-170.5 °C(lit.)
- Refractive Index:1.692
- Boiling Point:383.1 °C at 760 mmHg
- PKA:8.57±0.40(Predicted)
- Flash Point:185.5 °C
- PSA:44.02000
- Density:1.28 g/cm3
- LogP:2.41708
6-Cyano-2-naphthol(Cas 52927-22-7) Usage
|
General Description |
6-Cyano-2-naphthol (6CN2) is an aromatic alcohol that can be synthesized from 6-bromo-2-naphthol. It is a superphotoacid with the ground state pKa* value of 8.4 and excited state pKavalue of 0.2, respectively. 6CN2 protonates PANI-ES (polyaniline emeraldine salt) to form PANI-EB (emeraldine base), which shows enhanced conductivity. The proton-transfer kinetics and photophysical behavior of 6CN2 have been investigated. |
InChI:InChI=1/C11H7NO/c12-7-8-1-2-10-6-11(13)4-3-9(10)5-8/h1-6,13H
52927-22-7 Relevant articles
Process for Preparing Nafamostat Mesilate and Intermediate Thereof
-
Paragraph 0080-0081, (2021/10/27)
The present invention provides a method ...
A facile and versatile electro-reductive system for hydrodefunctionalization under ambient conditions
Huang, Binbin,Guo, Lin,Xia, Wujiong
supporting information, p. 2095 - 2103 (2021/03/26)
A general electrochemical system for red...
Decarboxylative Hydroxylation of Benzoic Acids
Ritter, Tobias,Su, Wanqi,Xu, Peng
supporting information, p. 24012 - 24017 (2021/10/06)
Herein, we report the first decarboxylat...
Iodine(III)-Mediated, Controlled Di- or Monoiodination of Phenols
Satkar, Yuvraj,Yera-Ledesma, Luisa F.,Mali, Narendra,Patil, Dipak,Segura-Quezada, Luis A.,Ramírez-Morales, Perla I.,Solorio-Alvarado, César R.,Navarro-Santos, Pedro
, p. 4149 - 4164 (2019/04/30)
An oxidative procedure for the electroph...
52927-22-7 Process route
-
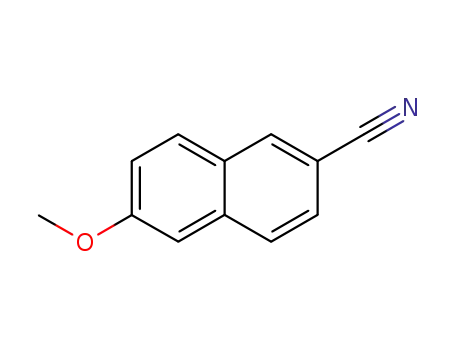
-
67886-70-8
2-cyano-6-methoxynaphthalene

-
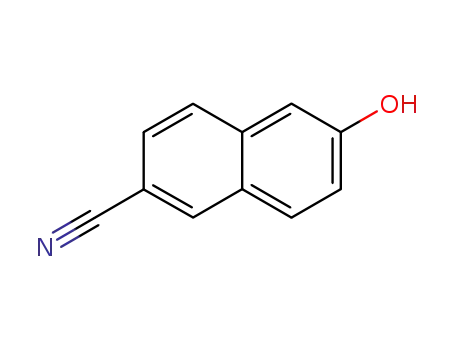
-
52927-22-7
6-cyano-2-naphthol
| Conditions | Yield |
|---|---|
|
|
91% |
|
With
tetrachlorosilane; borontrifluoride acetic acid; lithium iodide;
In
toluene; acetonitrile;
at 70 ℃;
for 10h;
|
83% |
|
With
boron tribromide;
In
dichloromethane;
at -78 - 20 ℃;
for 16h;
Inert atmosphere;
|
69% |
|
With
sodium cyanide;
In
dimethyl sulfoxide;
at 160 - 170 ℃;
for 12h;
|
62% |
|
With
boron tribromide;
|
-
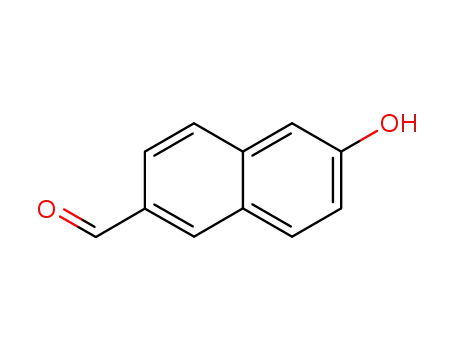
-
78119-82-1
6-hydroxynaphthalene-2-carbaldehyde

-

-
52927-22-7
6-cyano-2-naphthol
| Conditions | Yield |
|---|---|
|
With
acetic acid; hydroxylamine-O-sulfonic acid;
at 50 - 55 ℃;
for 5h;
Large scale;
|
85.7% |
|
With
hydroxylamine hydrochloride;
In
dimethyl sulfoxide;
at 100 ℃;
for 1h;
|
70% |
52927-22-7 Upstream products
-
860363-16-2
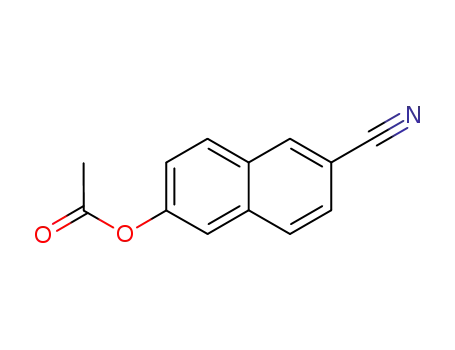
6-acetoxy-[2]naphthonitrile
-
15231-91-1
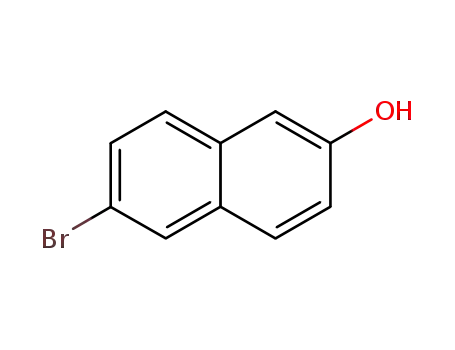
6-bromo-naphthalen-2-ol
-
78119-72-9
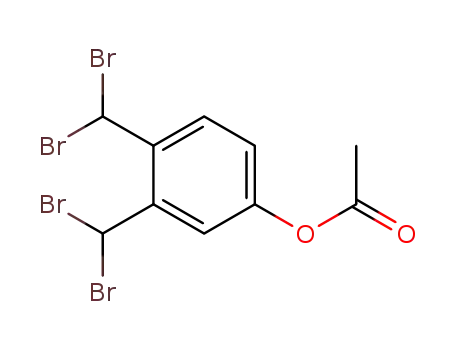
acetate of α,α,α',α'-tetrabromo-3,4-xylenol
-
107-13-1
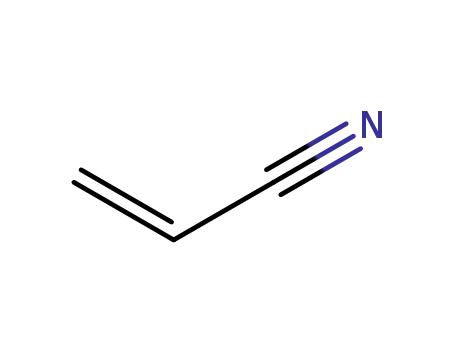
acrylonitrile
52927-22-7 Downstream products
-
82720-63-6

4-hexylphenylmethyl 6-cyano-2-naphthyl ether
-
58200-88-7
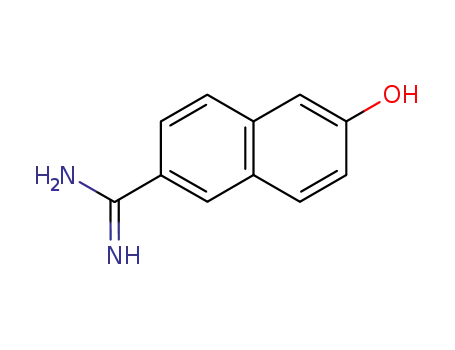
6-Amidino-2-naphthol
-
5159-60-4
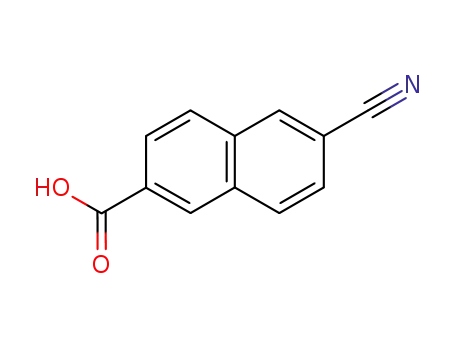
6-cyano-naphthalene-2-carboxylic acid
-
5088-91-5
6-cyano-naphthalene-2-carboxylic acid methyl ester
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
(R)-(+)-2-Tetrahydrofuroic acid
CAS:87392-05-0