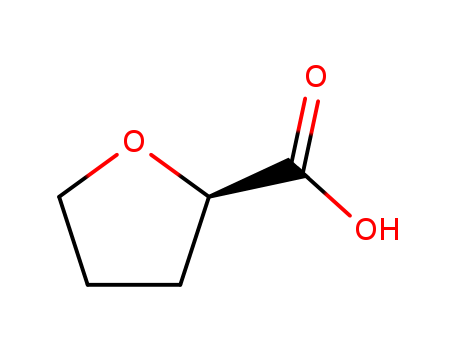
87392-05-0
- Product Name:(R)-(+)-2-Tetrahydrofuroic acid
- Molecular Formula:C5H8O3
- Purity:99%
- Molecular Weight:116.117
Product Details
pd_meltingpoint:128-129ºC 13 mm Hg(lit.)
Appearance:clear to pale yellow liquid
Top Quality Chinese Factory supply 87392-05-0 (R)-(+)-2-Tetrahydrofuroic acid
- Molecular Formula:C5H8O3
- Molecular Weight:116.117
- Appearance/Colour:clear to pale yellow liquid
- Vapor Pressure:0.0107mmHg at 25°C
- Melting Point:128-129ºC 13 mm Hg(lit.)
- Refractive Index:1.459
- Boiling Point:243.2 °C at 760 mmHg
- PKA:3.60±0.20(Predicted)
- Flash Point:113.2 °C
- PSA:46.53000
- Density:1.262 g/cm3
- LogP:0.25000
(R)-(+)-2-Tetrahydrofuroic acid(Cas 87392-05-0) Usage
|
Chemical Composition and Structure |
(R)-Tetrahydrofuran-2-carboxylic acid, also known as (R)-(+)-2-Tetrahydrofuroic acid, has the molecular structure of a tetrahydrofuran ring with a carboxylic acid functional group. |
|
History |
First incorporated into pharmaceutical synthesis in the mid-1980s as a key intermediate for furopenem. |
|
Production Methods |
Enzyme-catalyzed enantioselective hydrolysis of esters to produce (R)-Tetrahydrofuran-2-carboxylic acid.[2] |
InChI:InChI=1/C5H8O3/c6-5(7)4-2-1-3-8-4/h4H,1-3H2,(H,6,7)/t4-/m1/s1
87392-05-0 Relevant articles
Preparation process of optically pure 2-tetrahydrofuroic acid
-
Paragraph 0026; 0030; 0031, (2019/05/15)
The invention discloses a preparation pr...
Chiral ammonium hypoiodite salt-catalyzed enantioselective oxidative cycloetherification to 2-acyl tetrahydrofurans
Uyanik, Muhammet,Hayashi, Hiroki,Iwata, Hirokazu,Ishihara, Kazuaki
, p. 353 - 355 (2016/05/09)
2-Acyl tetrahydrofuran is a fundamental ...
Highly diastereoselective hydrogenation of furan-2-carboxylic acid derivatives on heterogeneous catalysts
Sebek, Michael,Holz, Jens,B?rner, Armin,J?hnisch, Klaus
experimental part, p. 461 - 465 (2009/08/09)
The heterogeneously catalyzed diastereos...
A scalable chemoenzymatic preparation of (R)-tetrahydrofuran-2-carboxylic acid
Fujima, Yoshito,Hirayama, Yoshihiro,Ikunaka, Masaya,Nishimoto, Yukifumi
, p. 1385 - 1391 (2007/10/03)
To develop a practical scalable approach...
87392-05-0 Process route
-
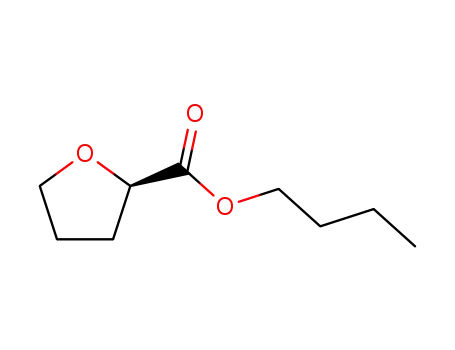
-
178461-69-3
butyl (R)-tetrahydrofuran-2-carboxylate

-
-
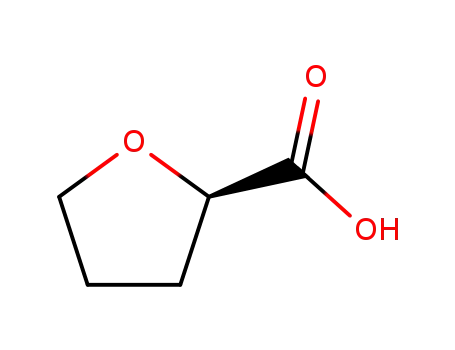
87392-05-0
(R)-tetrahydro-2-furoic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
tetrahydrofuran; methanol;
at 0 - 25 ℃;
for 0.5h;
|
-
-
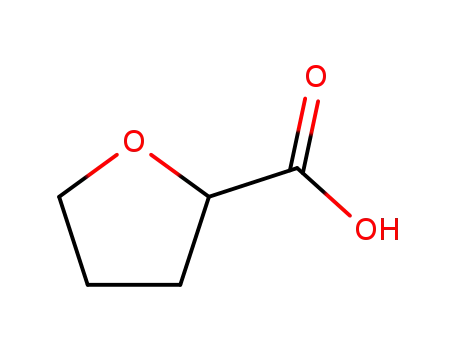
16874-33-2
tetrahydro-2-furancarboxylic acid

-
-
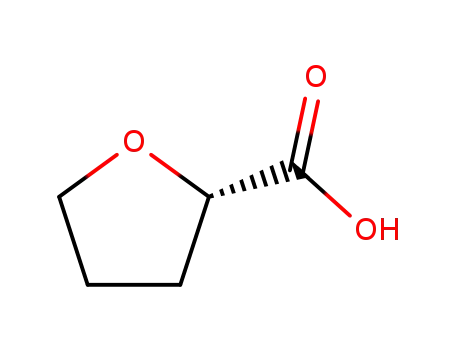
87392-07-2
(S)-tetrahydro-2-furoic acid

-

-
87392-05-0
(R)-tetrahydro-2-furoic acid
| Conditions | Yield |
|---|---|
|
With
L-Phenylalaninol;
In
ethyl acetate; acetone;
at 20 ℃;
for 1h;
|
9.339 g |
87392-05-0 Upstream products
-
97-99-4
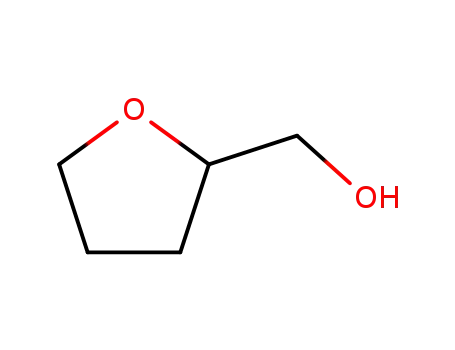
Tetrahydrofurfuryl alcohol
-
16874-33-2

tetrahydro-2-furancarboxylic acid
-
37443-42-8
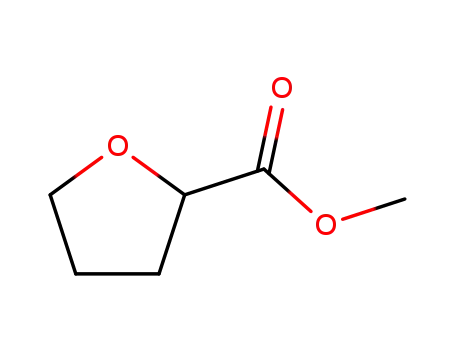
methyl tetrahydrofuran-2-carboxylate
-
16874-34-3
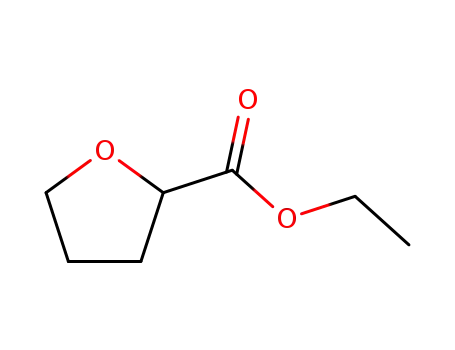
ethyl tetrahydrofurancarboxylate
87392-05-0 Downstream products
-
97-99-4
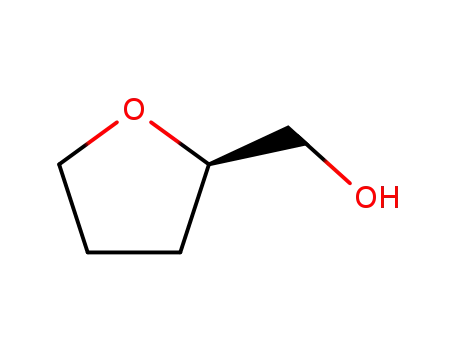
(R)-(tetrahydrofuran-2-yl)methanol
-
87324-00-3
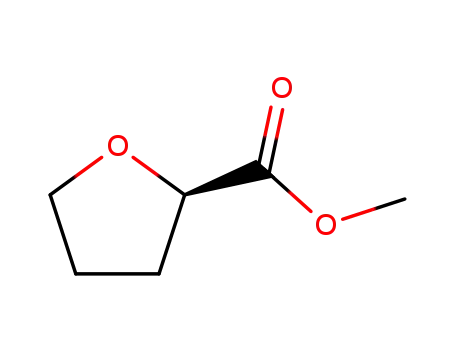
(R)-(-)-methyl tetrahydrofuran-2-carboxylate
-
444587-26-2
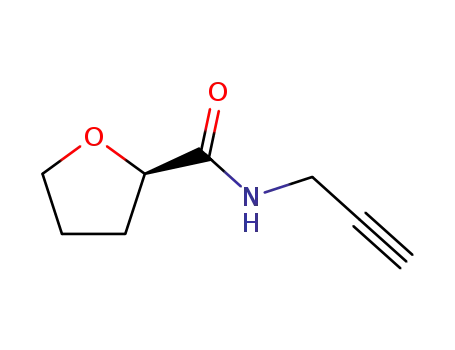
N-propargyl-(R)-(+)-tetrhydro-2-furoic amide
-
194089-81-1
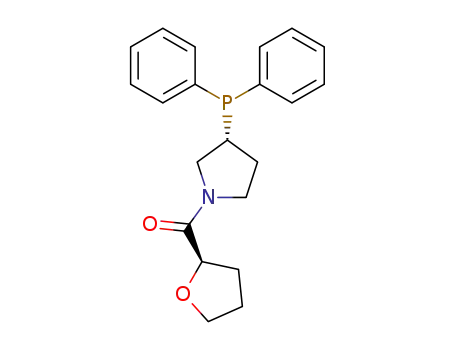
((R)-3-Diphenylphosphanyl-pyrrolidin-1-yl)-(R)-tetrahydro-furan-2-yl-methanone
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
6-Cyano-2-naphthol
CAS:52927-22-7