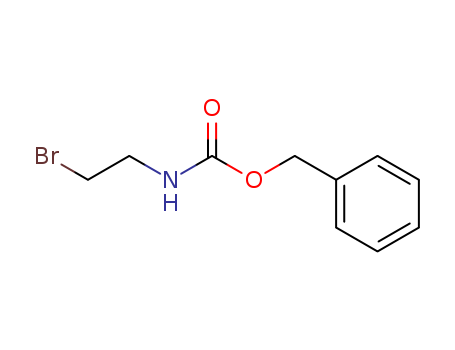
53844-02-3
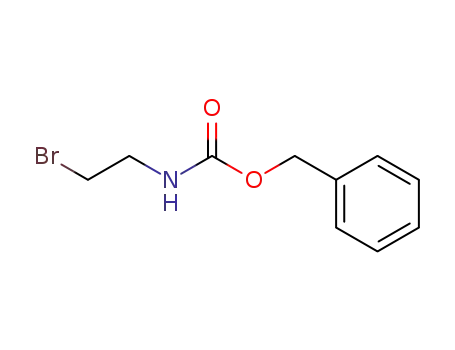
- Product Name:benzyl (2-bromoethyl)carbamate
- Molecular Formula:C10H12BrNO2
- Purity:99%
- Molecular Weight:258.115
Product Details
pd_meltingpoint:45 °C
China cas 53844-02-3 manufacturer wholesale benzyl (2-bromoethyl)carbamate at affordable price
- Molecular Formula:C10H12BrNO2
- Molecular Weight:258.115
- Vapor Pressure:2.12E-05mmHg at 25°C
- Melting Point:45 °C
- Refractive Index:1.556
- Boiling Point:361.1 °C at 760 mmHg
- PKA:11.55±0.46(Predicted)
- Flash Point:172.2 °C
- PSA:38.33000
- Density:1.428 g/cm3
- LogP:2.69860
BENZYL 2-BROMOETHYLCARBAMATE(Cas 53844-02-3) Usage
|
General Description |
Benzyl 2-Bromoethylcarbamate is a chemical compound with a molecular formula of C11H12BrNO2. BENZYL 2-BROMOETHYLCARBAMATE belongs to the carbamate esters category, a type of salts or esters of carbamic acid. The carbamate group comprises both an amine and a carbonyl functional group. Generally, carbamates are used in organizing industrial products like pharmaceuticals, biocides, and plasticizers. Nonetheless, data regarding specific properties, uses, or potential health effects of Benzyl 2-Bromoethylcarbamate is somewhat limited, indicating it may not be widely utilized or studied. |
InChI:InChI=1/C10H12BrNO2/c11-6-7-12-10(13)14-8-9-4-2-1-3-5-9/h1-5H,6-8H2,(H,12,13)
53844-02-3 Relevant articles
Chemoselective and Highly Sensitive Quantification of Gut Microbiome and Human Metabolites
Conway, Louis P.,Globisch, Daniel,L?hr, J.-Matthias,Lin, Weifeng,Vujasinovic, Miroslav
, p. 23232 - 23240 (2021)
The microbiome has a fundamental impact ...
Organic amine linking agent with three carbon-carbon double bonds and preparation method thereof
-
Paragraph 0030; 0066-0068; 0073, (2021/11/26)
The invention relates to the field of ol...
N-bromosuccinimide promoted synthesis of β-amino bromides under Appel reaction condition
Chinthaginjala, Srinivasulu,Alavandimat, Nanda H.,Umesha, Vathsala,Sureshbabu, Vommina V.
supporting information, p. 2975 - 2983 (2021/08/27)
An efficient and facile method has been ...
Rapid Assembly of Saturated Nitrogen Heterocycles in One-Pot: Diazo-Heterocycle “Stitching” by N–H Insertion and Cyclization
Boddy, Alexander J.,Affron, Dominic P.,Cordier, Christopher J.,Rivers, Emma L.,Spivey, Alan C.,Bull, James A.
supporting information, p. 1458 - 1462 (2019/01/04)
Methods that provide rapid access to new...
53844-02-3 Process route
-
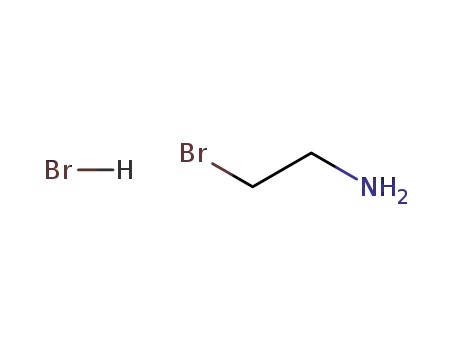
-
2576-47-8
2-bromoethylamine hydrobromide

-
-
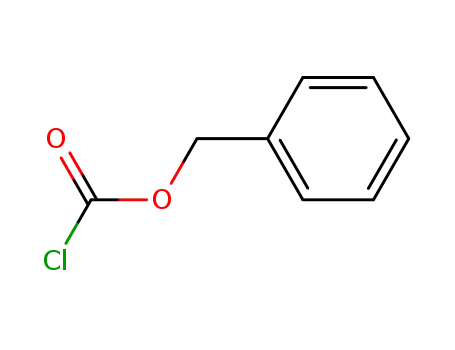
501-53-1,94274-21-2
benzyl chloroformate

-
-
53844-02-3
benzyl N-(2-bromoethyl)carbamate
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
Ambient temperature;
|
99% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 13.33h;
Inert atmosphere;
|
99% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 13.3333h;
Inert atmosphere;
|
99% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
96% |
|
With
potassium carbonate;
In
1,4-dioxane; water;
at 20 ℃;
for 1.5h;
pH=6 - 8;
|
95% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
|
95% |
|
With
sodium hydroxide;
In
1,4-dioxane;
Ambient temperature;
|
93% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
92% |
|
With
sodium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
|
90% |
|
With
triethylamine;
In
dichloromethane;
|
88% |
|
With
triethylamine;
In
ethanol;
at 20 ℃;
for 20h;
|
87% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
79% |
|
With
potassium carbonate;
In
1,4-dioxane; water;
pH=5 - 9;
Inert atmosphere;
|
75% |
|
With
sodium hydroxide;
for 0.5h;
|
66% |
|
With
N-ethyl-N,N-diisopropylamine;
In
DMF (N,N-dimethyl-formamide);
at 20 ℃;
for 2h;
|
64% |
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
64% |
|
|
|
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
|
|
With
sodium chloride; potassium carbonate;
In
tetrahydrofuran; water;
|
|
|
With
triethylamine;
In
dichloromethane;
|
|
|
With
potassium carbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 16h;
|
7.32 g |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 ℃;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 ℃;
|
|
|
2-bromoethylamine hydrobromide;
With
sodium hydroxide;
In
ethanol; water;
at 10 ℃;
pH=7.1;
benzyl chloroformate;
In
1,2-dimethoxyethane; ethanol; water;
at 20 ℃;
pH=7;
|
79.8 g |
|
With
sodium hydroxide;
In
1,2-dimethoxyethane; ethanol; water;
at 10 - 20 ℃;
for 18h;
pH=7 - 7.1;
|
79.8 g |
|
With
triethylamine;
In
chloroform;
at 0 - 20 ℃;
Cooling with ice;
|
|
|
With
potassium carbonate;
In
1,4-dioxane; water;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 8h;
Cooling with ice;
|
|
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
|
|
With
sodium hydroxide;
In
dichloromethane;
at 0 - 25 ℃;
for 2h;
pH=9;
|
-
-
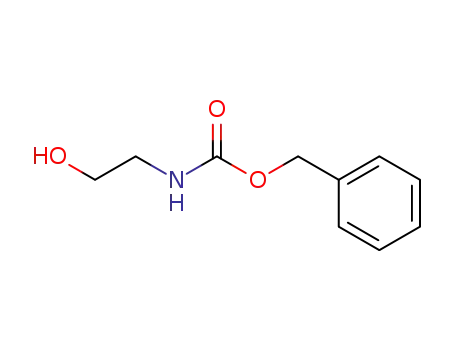
77987-49-6
benzyl 2-hydroxyethylcarbamate

-

-
53844-02-3
benzyl N-(2-bromoethyl)carbamate
| Conditions | Yield |
|---|---|
|
With
carbon tetrabromide; triphenylphosphine;
In
dichloromethane;
at 0 - 25 ℃;
for 24h;
|
95% |
|
With
N-Bromosuccinimide; triphenylphosphine;
In
dichloromethane;
at 0 - 20 ℃;
for 2.25h;
enantioselective reaction;
|
84% |
53844-02-3 Upstream products
-
107-09-5
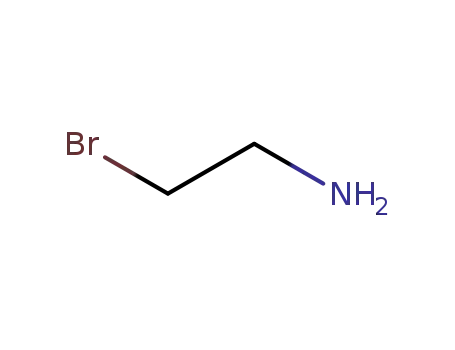
2-bromoethylamine
-
501-53-1

benzyl chloroformate
-
2304-94-1
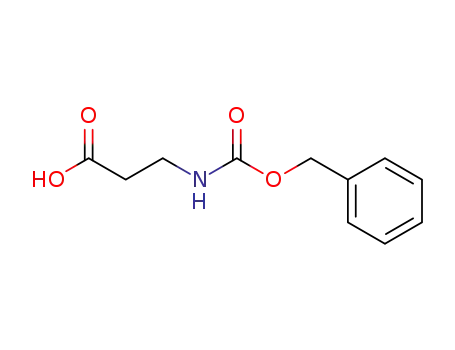
3-(benzyloxycarbonylamino)propanoic acid
-
2576-47-8
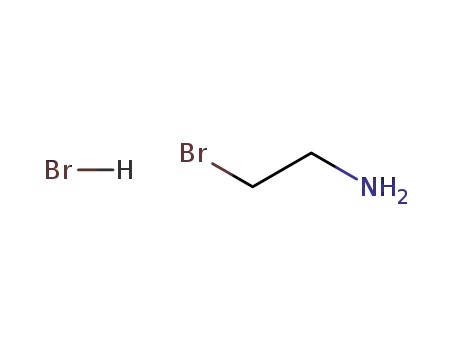
2-bromoethylamine hydrobromide
53844-02-3 Downstream products
-
82933-20-8
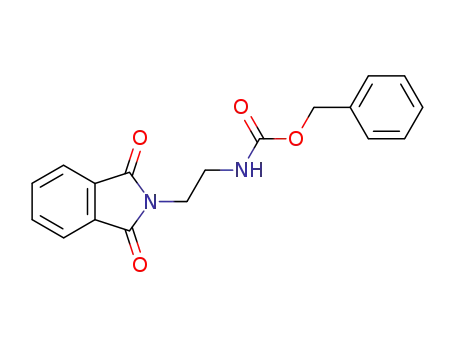
N-carbobenzoxy-N',N'-phthaloyl-1,3-diaminoethane
-
68373-12-6
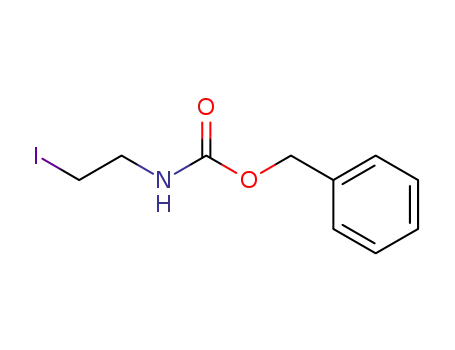
N-benzyloxycarbonyl-2-iodo-ethylamine
-
101288-83-9
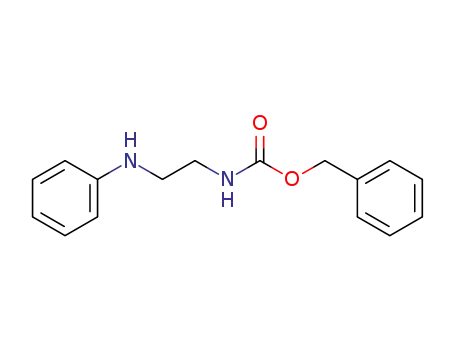
benzyl (2-(phenylamino)ethyl)carbamate
-
109960-08-9
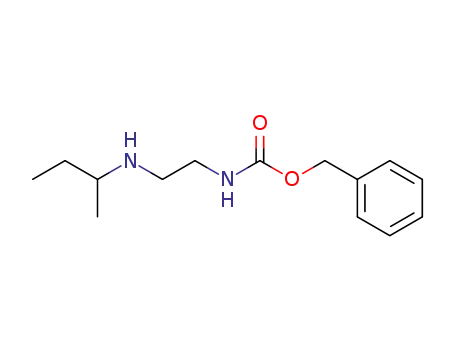
(+/-)-N-carbobenzoxy-2-<(1-methylpropyl)amino>ethylamine
Relevant Products
-
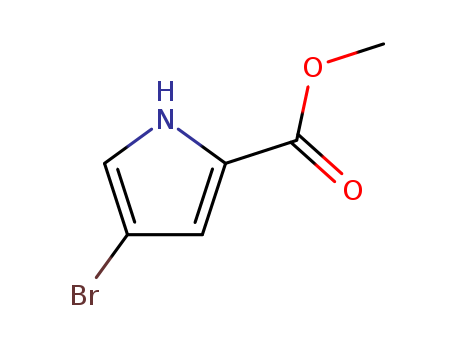
4-Bromo-2-(methoxycarbonyl)-1H-pyrrole
CAS:934-05-4
-
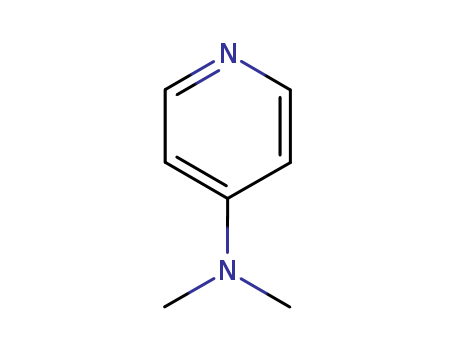
4-Dimethylaminopyridine
CAS:1122-58-3
-
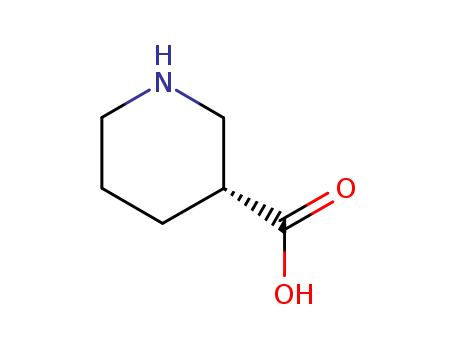
(S)-(+)-Nipecotic acid
CAS:59045-82-8