143322-57-0
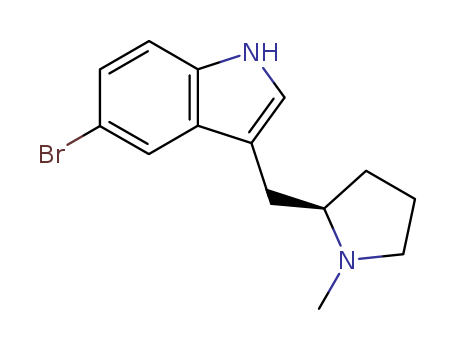
- Product Name:(R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole
- Molecular Formula:C14H17BrN2
- Purity:99%
- Molecular Weight:293.206
Product Details
Factory supply good quality (R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole 143322-57-0 with stock
- Molecular Formula:C14H17BrN2
- Molecular Weight:293.206
- Vapor Pressure:5E-07mmHg at 25°C
- Refractive Index:1.655
- Boiling Point:412.9 °C at 760 mmHg
- PKA:16.28±0.30(Predicted)
- Flash Point:203.5 °C
- PSA:19.03000
- Density:1.418 g/cm3
- LogP:3.50500
(R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole(Cas 143322-57-0) Usage
InChI:InChI=1/C14H17BrN2/c1-17-6-2-3-12(17)7-10-9-16-14-5-4-11(15)8-13(10)14/h4-5,8-9,12,16H,2-3,6-7H2,1H3/t12-/m1/s1
143322-57-0 Relevant articles
PROCESS FOR PREPARATION OF ELETRIPTAN AND SALT THEREOF
-
Page/Page column 12, (2012/04/04)
The present invention relates to an impr...
PROCESS FOR PREPARING PURE 5-BROMO-3-[(R)-1-METHYL-PYRROLIDIN-2-YLMETHYL]-1H-INDOLE, INTERMEDIATE FOR ELETRIPTAN
-
Page/Page column 5-6, (2012/03/26)
Disclosed is a process for preparing pur...
PROCESS FOR THE PREPARATION OF 5-SUBSTSITUTED INDOLE DERIVATIVE
-
, (2012/02/01)
The present invention relates to an impr...
SYNTHESIS OF 3--5-[2-(PHENYLSULFONYL)ETHYL]-1H-INDOLE
-
Page/Page column 4, (2011/07/29)
The present invention refers to the synt...
143322-57-0 Process route
-
![(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole](/upload/2024/12/347f5f48-f3a9-45f1-bde0-f63daa5116c1.png)
-
143322-56-9,331842-86-5
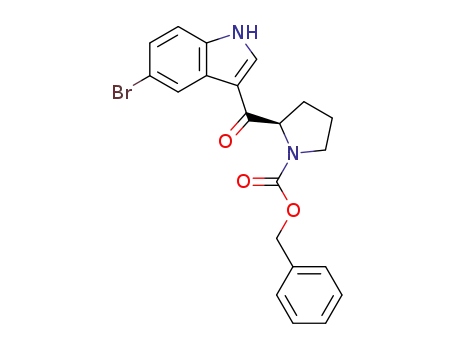
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole

-
-
143322-55-8
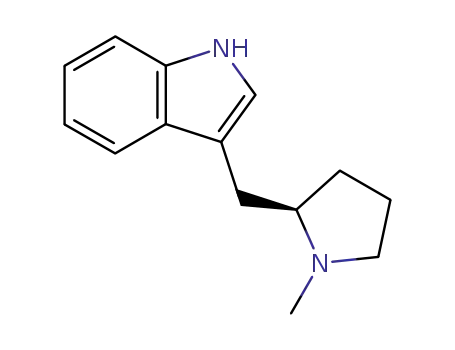
(R)-3-(1-methyl-2-pyrrolidinylmethyl)-1H-indole

-
-
C14H17BrN2O

-
-
180637-93-8
C14H15BrN2O

-
-
100-51-6,185532-71-2
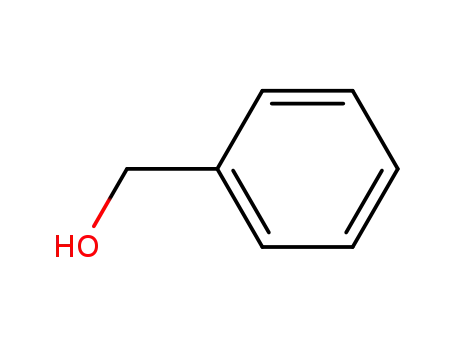
benzyl alcohol

-
-
143322-57-0,208464-41-9,312949-16-9
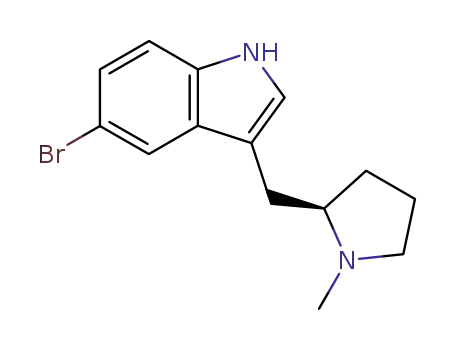
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole
| Conditions | Yield |
|---|---|
|
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole;
With
sodium bis(2-methoxyethoxy)aluminium dihydride;
In
toluene;
at 30 - 48 ℃;
for 2.66667h;
With
sodium hydroxide; water;
In
toluene;
at 15 - 20 ℃;
for 1h;
Product distribution / selectivity;
|
57% |
|
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole;
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 20 ℃;
for 39h;
Heating / reflux;
With
sodium hydroxide; water;
In
tetrahydrofuran;
for 0.5h;
Product distribution / selectivity;
|
47% |
-
![5-bromo-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole ethanedioate](/upload/2024/12/0ca71893-435e-4a9e-9702-a59505fa9982.png)
-
1196663-29-2
5-bromo-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole ethanedioate

-

-
143322-57-0,208464-41-9,312949-16-9
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
water; toluene;
at 30 - 35 ℃;
for 0.5h;
pH=7.8;
|
90.5% |
|
With
potassium hydroxide;
In
water; toluene;
at 21 - 25 ℃;
for 0.25h;
|
|
|
With
potassium carbonate;
In
water;
pH=7;
|
143322-57-0 Upstream products
-
143322-56-9
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole
-
1196663-29-2
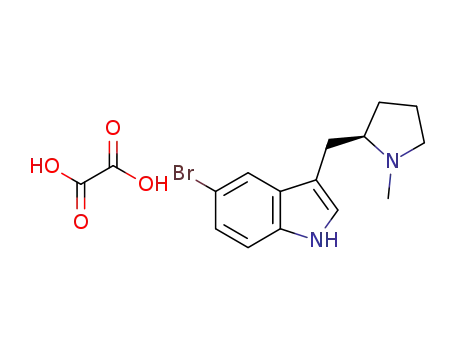
5-bromo-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole ethanedioate
-
6404-31-5
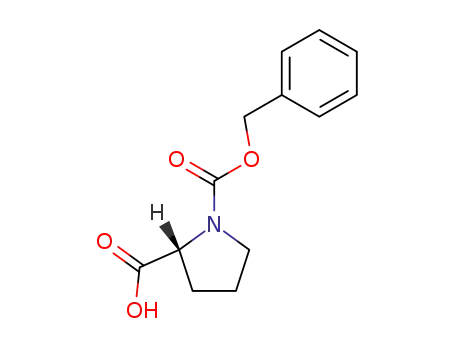
Z-D-proline
-
61350-62-7
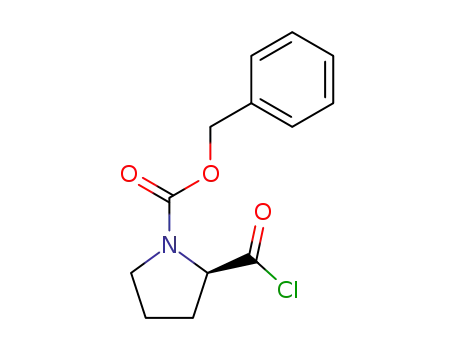
N-benzyloxycarbonyl-D-proline acid chloride
143322-57-0 Downstream products
-
205369-12-6
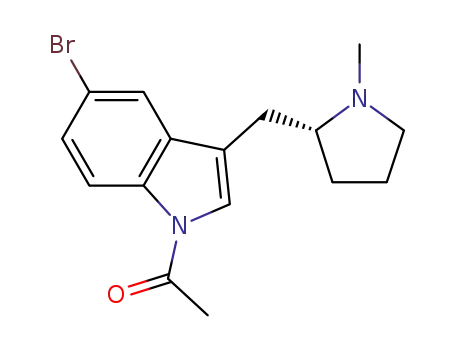
(R)-1-acetyl-5-bromo-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole
-
180637-88-1
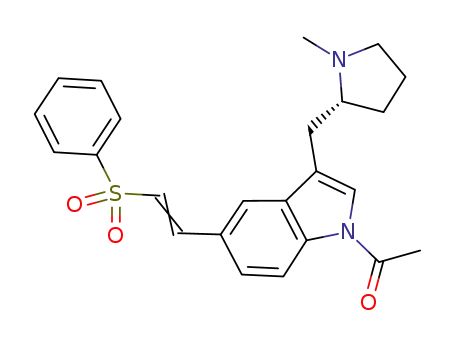
(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole
-
208464-46-4
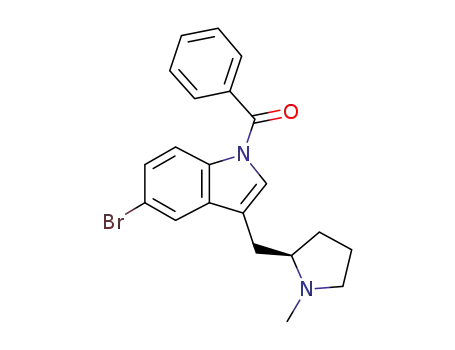
(R)-5-bromo-3-[(N-methylpyrrolidin-2-yl)methyl]-1-benzoylindole
Relevant Products
-
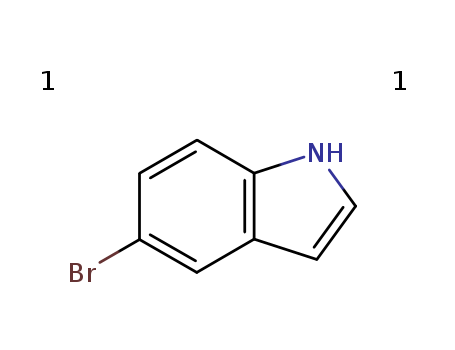
5-Bromoindole
CAS:10075-50-0
-
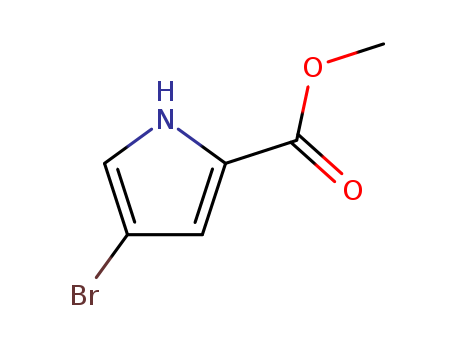
4-Bromo-2-(methoxycarbonyl)-1H-pyrrole
CAS:934-05-4
-
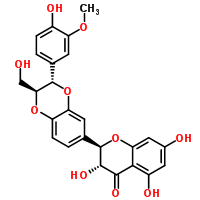
Silymarin
CAS:65666-07-1