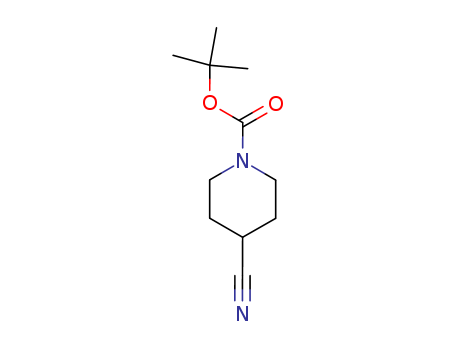
91419-52-2
- Product Name:1-Boc-4-Cyanopiperidine
- Molecular Formula:C11H18N2O2
- Purity:99%
- Molecular Weight:210.276
Product Details
pd_meltingpoint:60-62 °C
Appearance:yellowish oil
China cas 91419-52-2 factory wholesale 1-Boc-4-Cyanopiperidine at affordable price
- Molecular Formula:C11H18N2O2
- Molecular Weight:210.276
- Appearance/Colour:yellowish oil
- Vapor Pressure:0mmHg at 25°C
- Melting Point:60-62 °C
- Refractive Index:1.488
- Boiling Point:325.3 °C at 760 mmHg
- PKA:-3.08±0.40(Predicted)
- Flash Point:150.5 °C
- PSA:53.33000
- Density:1.07 g/cm3
- LogP:2.09498
1-Boc-4-cyanopiperidine(Cas 91419-52-2) Usage
InChI:InChI=1/C11H18N2O2/c1-11(2,3)15-10(14)13-6-4-9(8-12)5-7-13/h9H,4-7H2,1-3H3
91419-52-2 Relevant articles
Sustainable Route Toward N-Boc Amines: AuCl3/CuI-Catalyzed N-tert-butyloxycarbonylation of Amines at Room Temperature
Cao, Yanwei,Huang, Yang,He, Lin
, (2021/12/22)
N-tert-butoxycarbonyl (N-Boc) amines are...
Nickel-Catalyzed Deaminative Cyanation: Nitriles and One-Carbon Homologation from Alkyl Amines
Xu, Jianyu,Twitty, J. Cameron,Watson, Mary P.
supporting information, p. 6242 - 6245 (2021/08/23)
A nickel-catalyzed deaminative cyanation...
Nickel-Catalyzed Cyanation of Unactivated Alkyl Sulfonates with Zn(CN)2
Xia, Aiyou,Lv, Peizhuo,Xie, Xin,Liu, Yuanhong
supporting information, p. 7842 - 7847 (2020/11/02)
Cyanation of unactivated primary and sec...
Synthesis of 1,4- and 1,4,4-substituted piperidines for the inhibition of neuronal T-type Ca2+ channels and mitigation of neuropathic pain in mice
Gunaratna, Medha J.,Hua, Duy H.,Zou, Bende,Pascual, Conrado,Cao, William,Zhang, Man,Weerasekara, Sahani,Nguyen, Thi D.T.,Xiao, Kui,Xie, Xinmin Simon
, p. 22 - 39 (2019/03/08)
A dysregulation in function of neuronal ...
91419-52-2 Process route
-
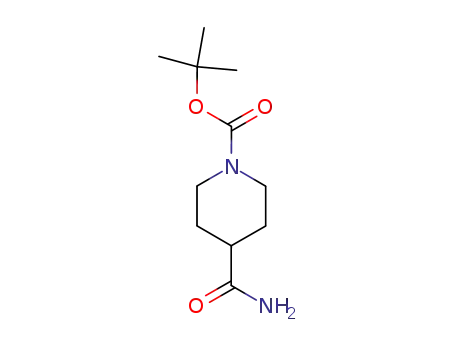
-
91419-48-6
4-carbamoyl-piperidine-1-carboxylic acid tert-butyl ester

-
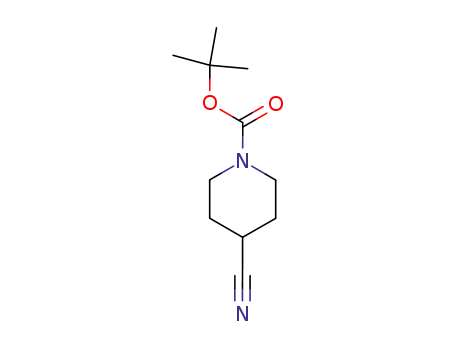
-
91419-52-2
1-tert-butoxycarbonyl-4-cyanopiperidine
| Conditions | Yield |
|---|---|
|
With
triethylamine; ethanaminium,N-(difluoro-λ4-sulfanylidene)-N-ethyl-,tetrafluoroborate;
In
ethyl acetate;
at 20 ℃;
for 1h;
Inert atmosphere;
|
98% |
|
4-carbamoyl-piperidine-1-carboxylic acid tert-butyl ester;
With
triethylamine; trichlorophosphate;
In
dichloromethane;
at 0 - 20 ℃;
With
sodium hydrogencarbonate;
In
dichloromethane;
|
70% |
|
With
triethylamine; trichlorophosphate;
In
dichloromethane;
at 0 ℃;
|
69% |
|
With
trichlorophosphate;
In
pyridine;
Yield given;
|
|
|
With
trifluoroacetic anhydride;
In
pyridine; dichloromethane;
at 20 ℃;
for 2h;
|
7.37 g |
|
With
triethylamine; trifluoroacetic anhydride;
In
dichloromethane;
at 20 ℃;
for 3.5h;
|
|
|
With
triphenylphosphine;
In
tetrahydrofuran; tetrachloromethane;
|
|
|
With
diethylamine; trifluoroacetic anhydride;
In
tetrahydrofuran;
at 0 ℃;
Inert atmosphere;
|
|
|
With
triethylamine; trifluoroacetic anhydride;
In
dichloromethane;
at 0 - 23 ℃;
for 5h;
Inert atmosphere;
|
1.29 g |
-
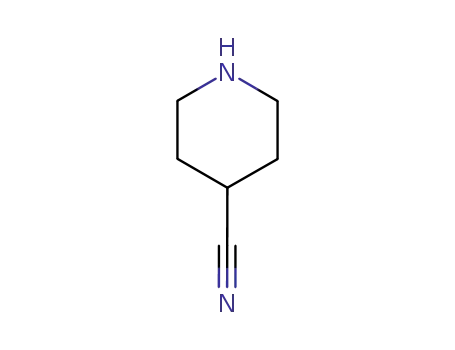
-
4395-98-6
4-cyanopiperidine

-
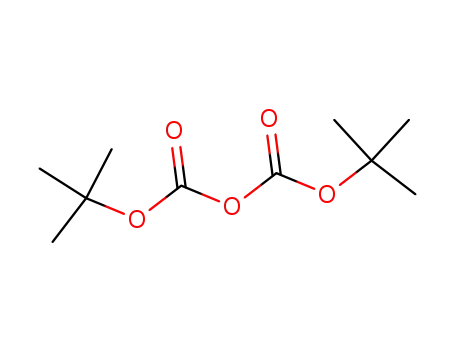
-
24424-99-5
di-tert-butyl dicarbonate

-

-
91419-52-2
1-tert-butoxycarbonyl-4-cyanopiperidine
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 12h;
Inert atmosphere;
|
100% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 25 ℃;
for 12h;
Inert atmosphere;
|
100% |
|
In
1,4-dioxane;
at 20 ℃;
|
97% |
|
In
1,4-dioxane;
at 20 ℃;
|
97% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
96% |
|
In
dichloromethane;
for 15.25h;
|
96% |
|
In
dichloromethane;
at 25 ℃;
for 1h;
|
83% |
|
With
sodium hydrogencarbonate;
at 20 ℃;
for 20h;
|
79% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 1h;
|
73.6% |
|
With
sodium hydrogencarbonate;
In
water;
|
43% |
|
With
potassium hydrogensulfate;
In
dichloromethane; water; ethyl acetate;
|
|
|
In
methanol;
|
13.4 g (80%) |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
In
methanol;
for 5h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
sodium hydroxide;
In
diethyl ether; water;
at 0 - 20 ℃;
for 12h;
|
|
|
In
1,4-dioxane;
at 20 ℃;
for 16h;
|
91419-52-2 Upstream products
-
4395-98-6

4-cyanopiperidine
-
1538-75-6
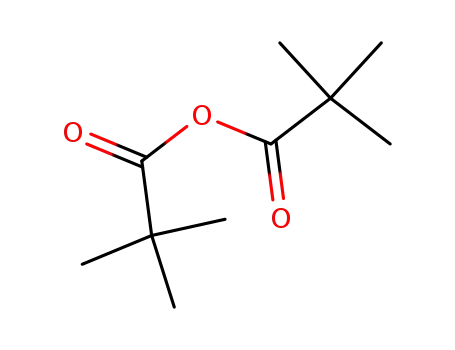
2,2-dimethylpropanoic anhydride
-
91419-48-6

4-carbamoyl-piperidine-1-carboxylic acid tert-butyl ester
-
24424-99-5

di-tert-butyl dicarbonate
91419-52-2 Downstream products
-
91419-58-8
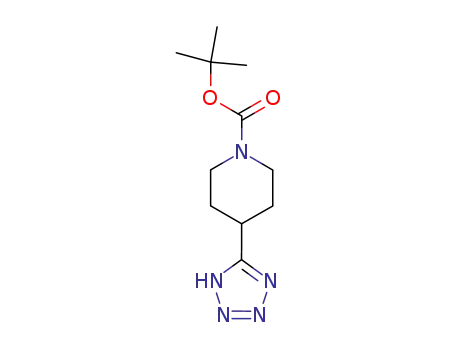
tert-butyl 4-(1H-tetrazol-5-yl)piperidine-1-carboxylate
-
206989-61-9
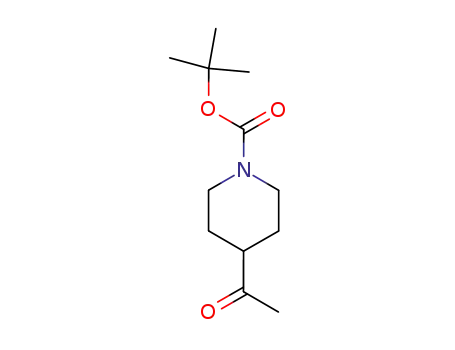
4-acetyl-1-(t-butoxycarbonyl)piperidine
-
4395-98-6

4-cyanopiperidine
-
269741-29-9
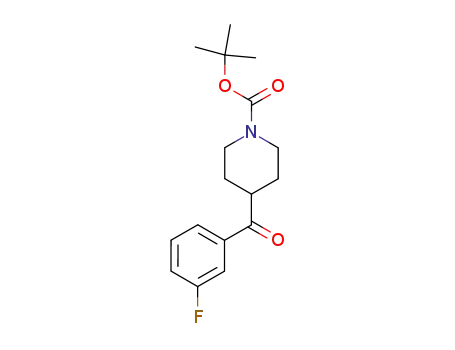
4-(3-fluorobenzoyl)piperidine-1-carboxylic acid tert-butyl ester
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
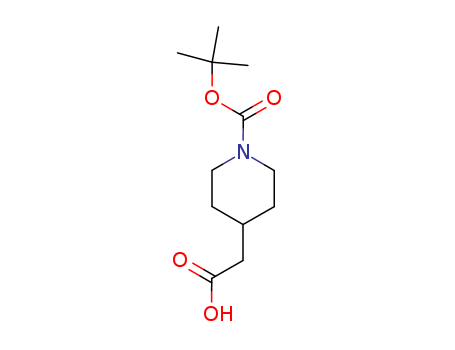
N-Boc-4-piperidineacetic acid
CAS:157688-46-5
-
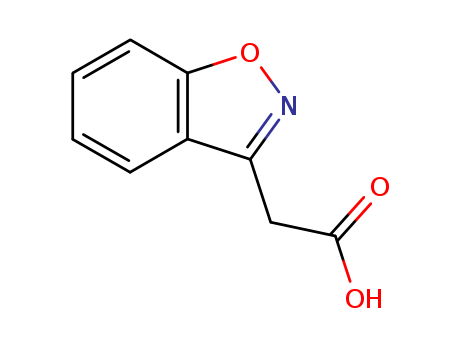
2-(1,2-Benzisoxazol-3-yl)acetic acid
CAS:4865-84-3