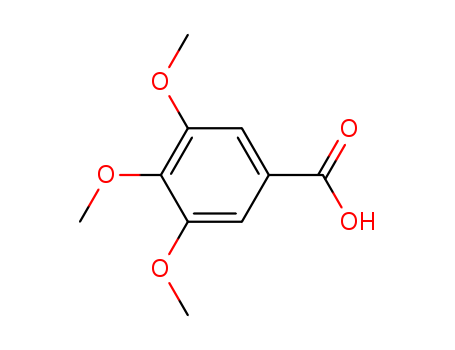
118-41-2
- Product Name:3,4,5-Trimethoxybenzoic acid
- Molecular Formula:C10H12O5
- Purity:99%
- Molecular Weight:212.202
Product Details
pd_meltingpoint:168-171 °C(lit.)
Appearance:white to beige fine crystalline powder
Reputable factory supply 3,4,5-Trimethoxybenzoic acid 118-41-2 in bulk at low price
- Molecular Formula:C10H12O5
- Molecular Weight:212.202
- Appearance/Colour:white to beige fine crystalline powder
- Vapor Pressure:2.49E-06mmHg at 25°C
- Melting Point:168-171 °C(lit.)
- Refractive Index:1.5140 (estimate)
- Boiling Point:376.3 °C at 760 mmHg
- PKA:4.23±0.10(Predicted)
- Flash Point:128.8 °C
- PSA:64.99000
- Density:1.219 g/cm3
- LogP:1.41060
Gallic acid trimethyl ether(Cas 118-41-2) Usage
|
Synthesis |
3,4,5-trimethoxybenzoic acid can be synthesised by methoxylation of gallic acid and dimethyl sulfate, and the fine product is obtained after neutralization, filtration and washing. |
|
Physical properties |
3,4,5-Trimethoxy benzoic acid is a white to beige fine crystalline powder. |
|
Definition |
ChEBI: A benzoic acid derivative carrying 3-, 4- and 5-methoxy substituents. |
InChI:InChI=1/C10H12O5/c1-13-7-4-6(10(11)12)5-8(14-2)9(7)15-3/h4-5H,1-3H3,(H,11,12)/p-1
118-41-2 Relevant articles
Cloning and heterologous expression of two aryl-aldehyde dehydrogenases from the white-rot basidiomycete Phanerochaete chrysosporium
Nakamura, Tomofumi,Ichinose, Hirofumi,Wariishi, Hiroyuki
, p. 470 - 475 (2010)
We identified two aryl-aldehyde dehydrog...
Cytotoxic cholestane glycosides from the bulbs of Ornithogalum saundersiae
Kuroda, Minpei,Mimaki, Yoshihiro,Yokosuka, Akihito,Sashida, Yutaka,Beutler, John A.
, p. 88 - 91 (2001)
Further phytochemical analysis of the bu...
One pot synthesis of α-aminophosphonates containing bromo and 3,4,5-trimethoxybenzyl groups under solvent-free conditions
Li, Caihong,Song, Baoan,Yan, Kai,Xu, Gangfang,Hu, Deyu,Yang, Song,Jin, Linhong,Xue, Wei,Lu, Ping
, p. 163 - 172 (2007)
New α-aminophosphonates were synthesized...
ALSTOLENINE, 19,20-DIHYDROPOLYNEURIDINE AND OTHER MINOR ALKALOIDS OF THE LEAVES OF ALSTONIA VENENATA
Majumder, Priyalal,Basu, Ashoke
, p. 2389 - 2392 (1982)
Structures of alstolenine and 19,20-dihy...
New flavones from Bauhinia championii Benth
Chen,Chen,Hsu,Chen
, p. 166 - 169 (1984)
-
-
Buziashvili et al.
, (1973)
-
-
Herzig,Tscherne
, p. 991 (1905)
-
-
Nierenstein
, p. 4012 (1930)
-
The Selective Liquid-Phase Oxidation of 3,4,5-Trimethoxytoluene to 3,4,5-Trimethoxybezaldehyde
Kitajima, Nobumasa,Takemura, Kazuya,Moro-Oka, Yoshihiko,Yoshikuni, Tadatsugu,Akada, Mitsuo,et al.
, p. 1035 - 1038 (1988)
The selective liquid-phase oxidation of ...
-
Roberts,Myers
, p. 153,155 (1960)
-
Solvolysis, Electrochemistry, and Development of Synthetic Building Blocks from Sawdust
Nguyen, Bichlien H.,Perkins, Robert J.,Smith, Jake A.,Moeller, Kevin D.
, p. 11953 - 11962 (2015)
Either aldehyde or cinnamyl ether produc...
-
Kamilov et al.
, (1976)
-
Synthesis and antitumor activity of novel 6,7,8-trimethoxy N-aryl-substituted-4-aminoquinazoline derivatives
Liu, Fang,Huai, Ziyou,Xia, Guotai,Song, Liuping,Li, Sha,Xu, Yulan,Hong, Kangjun,Yao, Mingyue,Liu, Gang,Huang, Yinjiu
, p. 2561 - 2565 (2018)
A series of 6,7,8-trimethoxy N-aryl-subs...
-
Gadamer
, (1897)
-
-
Saidkhodzhaev et al.
, (1978)
-
-
Herzig
, p. 846,847 (1912)
-
-
Subramanian,Nair
, p. 1679 (1971)
-
One-Pot Biocatalytic In Vivo Methylation-Hydroamination of Bioderived Lignin Monomers to Generate a Key Precursor to L-DOPA
Birmingham, William R.,Galman, James L.,Parmeggiani, Fabio,Seibt, Lisa,Turner, Nicholas J.
, (2022/01/13)
Electron-rich phenolic substrates can be...
Polyhydroxybenzoic acid derivatives as potential new antimalarial agents
Degotte, Gilles,Francotte, Pierre,Pirotte, Bernard,Frédérich, Michel
, (2021/08/07)
With more than 200 million cases and 400...
Synthesis and antioxidant activities of berberine 9-: O -benzoic acid derivatives
Liu, Yanfei,Long, Shuo,Zhang, Shanshan,Tan, Yifu,Wang, Ting,Wu, Yuwei,Jiang, Ting,Liu, Xiaoqin,Peng, Dongming,Liu, Zhenbao
, p. 17611 - 17621 (2021/05/29)
Although berberine (BBR) shows antioxida...
Selectively Upgrading Lignin Derivatives to Carboxylates through Electrochemical Oxidative C(OH)?C Bond Cleavage by a Mn-Doped Cobalt Oxyhydroxide Catalyst
Zhou, Hua,Li, Zhenhua,Xu, Si-Min,Lu, Lilin,Xu, Ming,Ji, Kaiyue,Ge, Ruixiang,Yan, Yifan,Ma, Lina,Kong, Xianggui,Zheng, Lirong,Duan, Haohong
supporting information, p. 8976 - 8982 (2021/03/16)
Oxidative cleavage of C(OH)?C bonds to a...
118-41-2 Process route
-
-
1207605-84-2
C20H23NO4

-
-
491-35-0
C6H4NCH2CHCCH2

-
-
1370460-33-5
C20H22N2O5

-
-
118-41-2
Eudesmic acid
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid; sodium nitrite;
In
chloroform;
at 0 - 5 ℃;
for 1h;
|
46% |
-
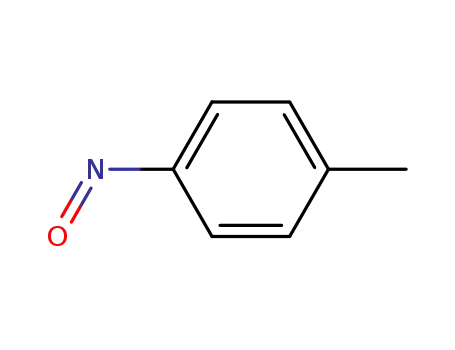
-
623-11-0
1-methyl-4-nitrosobenzene

-
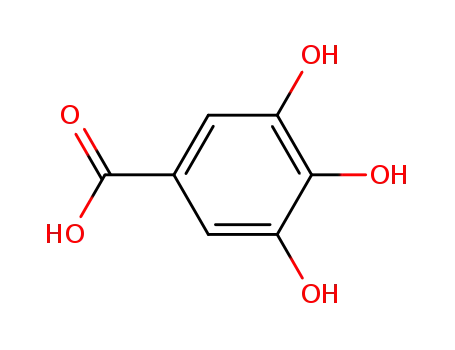
-
149-91-7
3,4,5-trihydroxybenzoic acid

-
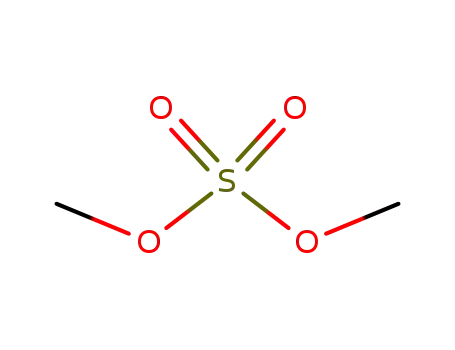
-
77-78-1
dimethyl sulfate

-
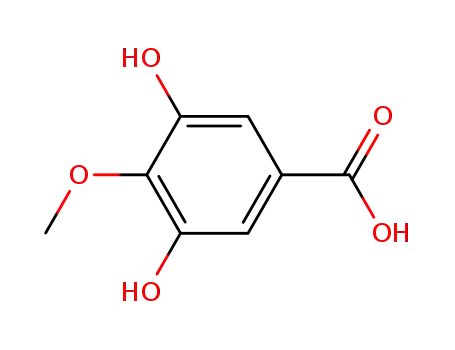
-
4319-02-2
4-O-methylgallic acid

-

-
118-41-2
Eudesmic acid
| Conditions | Yield |
|---|---|
|
|
118-41-2 Upstream products
-
186581-53-3

diazomethane
-
6635-24-1
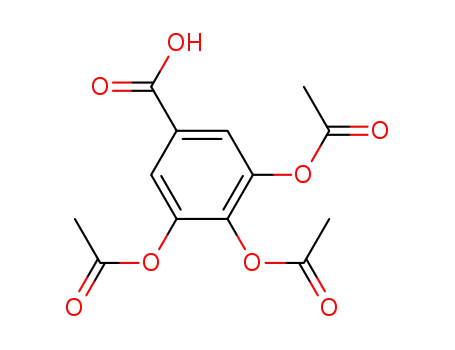
3,4,5-triacetoxybenzoic acid
-
54-04-6
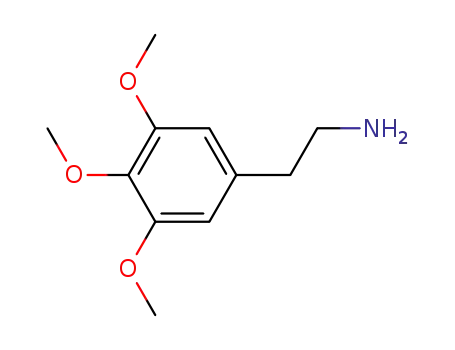
mescaline
-
90-50-6
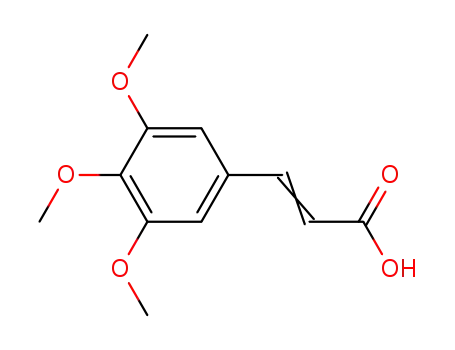
3,4,5-trimethoxycinnamic acid
118-41-2 Downstream products
-
1916-07-0
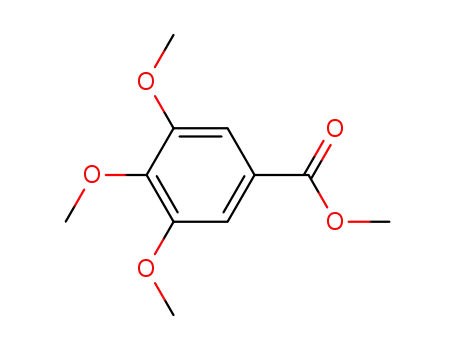
3,4,5-trimethoxybenzoic acid methyl ester
-
101889-07-0
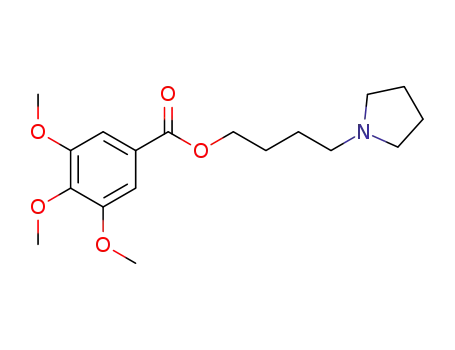
4-(pyrrolidinobutyl)3,4,5-trimethoxybenzoate
-
108369-31-9
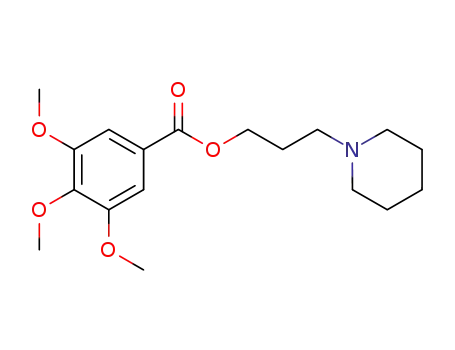
3,4,5-trimethoxy-benzoic acid-(3-piperidino-propyl ester)
-
99066-39-4
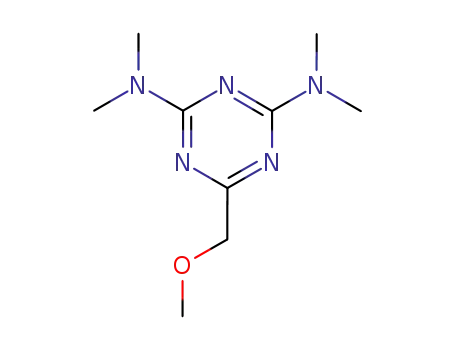
6-methoxymethyl-N2,N2,N4,N4-tetramethyl-[1,3,5]triazine-2,4-diyldiamine
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
2-Acrylamide-2-methylpropanesulfonic acid (AMPS)
CAS:15214-89-8
-
(R)-1-Boc-3-hydroxymethylpiperazine
CAS:278788-66-2