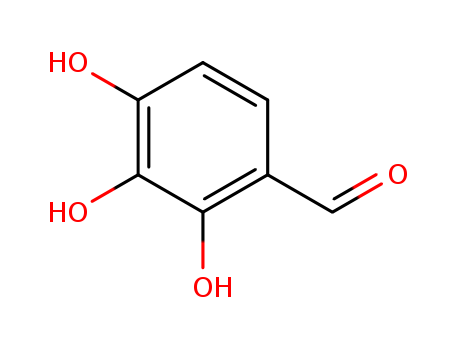
2144-08-3
- Product Name:2,3,4-Trihydroxybenzaldehyde
- Molecular Formula:C7H6O4
- Purity:99%
- Molecular Weight:154.122
Product Details
pd_meltingpoint:159-161 °C(lit.)
Appearance:light brown to beige-brown powder
Factory Sells Best Quality 2,3,4-Trihydroxybenzaldehyde 2144-08-3 with USP
- Molecular Formula:C7H6O4
- Molecular Weight:154.122
- Appearance/Colour:light brown to beige-brown powder
- Vapor Pressure:0.000573mmHg at 25°C
- Melting Point:159-161 °C(lit.)
- Refractive Index:1.734
- Boiling Point:301.9 °C at 760 mmHg
- PKA:7.41±0.23(Predicted)
- Flash Point:150.6 °C
- PSA:77.76000
- Density:1.598 g/cm3
- LogP:0.61590
2,3,4-Trihydroxybenzaldehyde(Cas 2144-08-3) Usage
|
General Description |
Antimicrobial activity of carbohydrazone derived from 2,3,4-trihydroxybenzaldehyde against bacteria and fungi has been investigated. 2,3,4-trihydroxybenzaldehyde forms Schiff bases via [1+1] condensation with anthranilic acid. |
InChI:InChI=1/C7H6O4/c8-3-4-1-2-5(9)7(11)6(4)10/h1-3,9-11H
2144-08-3 Relevant articles
Synthesis method of 2,3,4-trihydroxyl benzaldehyde
-
Paragraph 0030-0034, (2021/05/26)
The invention provides a synthesis metho...
Dienaminodiones, new push-pull alkenes, from 3,4-dihydroxysalicylaldehyde-derived Schiff base
Ravindran, Jaice,Ingaladal, Nagaraja,Lankalapalli, Ravi S.
supporting information, (2020/10/20)
An atom-economical one-pot synthesis of ...
Benzyl and benzaldehyde-derived metabolites from desert actinomycetes Sp. CDS
Nazir, Mamona,Mustafa, Rizwana,Tousif, Muhammad Imran,Ahmad, Shabir,Khatoon, Tasneem,Ahmad, Ishtiaq
, p. 919 - 923 (2018/10/02)
Chromatographic purification of the cult...
Ubiquinones and related compounds. XXII. Synthesis of 2,3-dimethoxy-5-methyl-1,4-benzoquinone and its ethylhomologues
Sugihara,Watanabe,Kawamatsu,Morimoto
, p. 109 - 120 (2007/10/05)
-
2144-08-3 Process route
-
-
109103-74-4
2,3,4-Trihydroxy-benzaldehyd-p-tolylimin

-
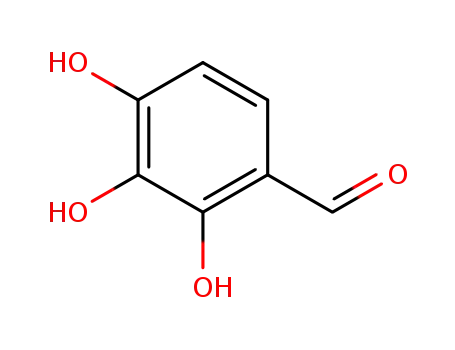
-
2144-08-3
2,3,4-trihydroxybenzylaldehyde

-
-
106-49-0,12221-03-3
p-toluidine
| Conditions | Yield |
|---|---|
|
With
methanol; sodium tetrahydroborate;
|
|
|
Multi-step reaction with 2 steps
1: [bis(acetoxy)iodo]benzene
2: sodium tetrahydroborate; methanol
With
methanol; sodium tetrahydroborate; [bis(acetoxy)iodo]benzene;
|
-
-
C14H11NO3

-

-
2144-08-3
2,3,4-trihydroxybenzylaldehyde

-
-
106-49-0,12221-03-3
p-toluidine
| Conditions | Yield |
|---|---|
|
With
methanol; sodium tetrahydroborate;
|
2144-08-3 Upstream products
-
864131-95-3
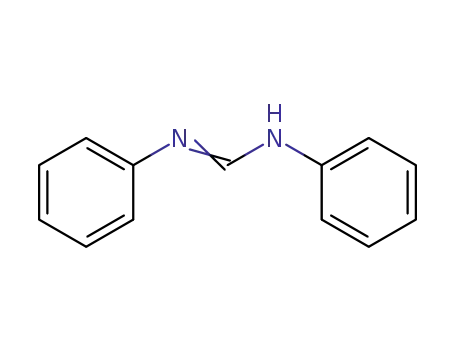
N,N'-diphenylformamidine
-
87-66-1
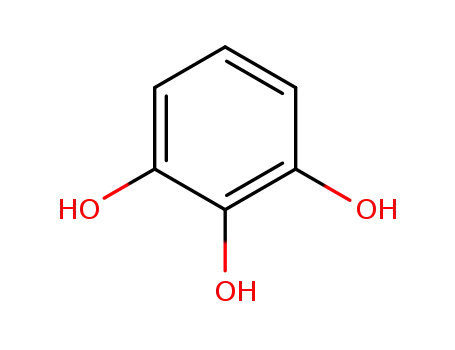
2-hydroxyresorcinol
-
103-70-8
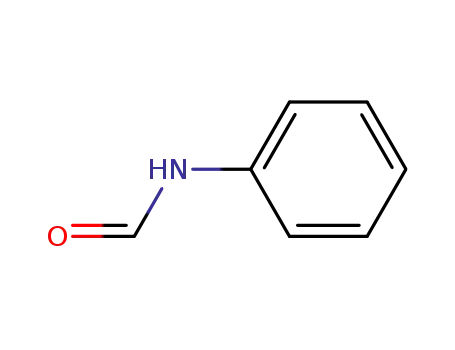
Formanilid
-
74-90-8

hydrogen cyanide
2144-08-3 Downstream products
-
65618-22-6
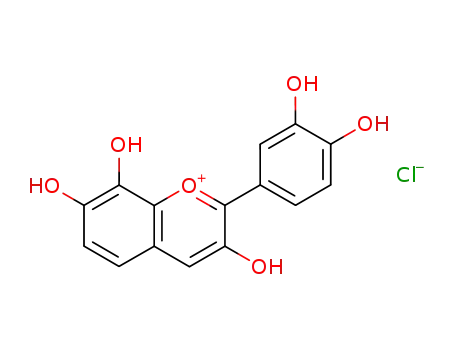
2-(3,4-dihydroxy-phenyl)-3,7,8-trihydroxy-chromenylium; chloride
-
64-18-6
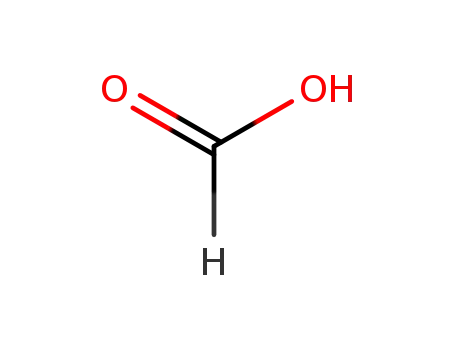
formic acid
-
857783-33-6
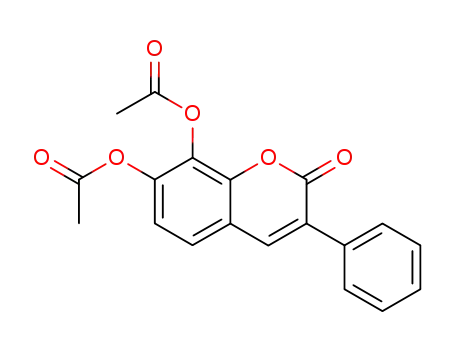
7,8-diacetoxy-3-phenylcoumarin
-
100727-75-1
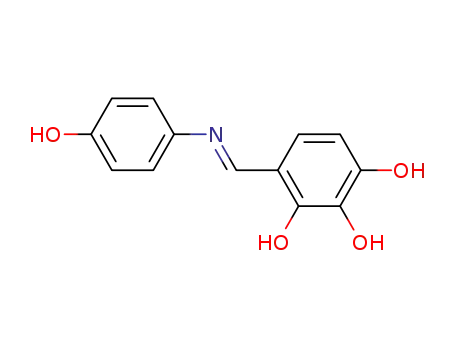
N-(2,3,4-trihydroxy-benzylidene)-p-hydroxyaniline
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
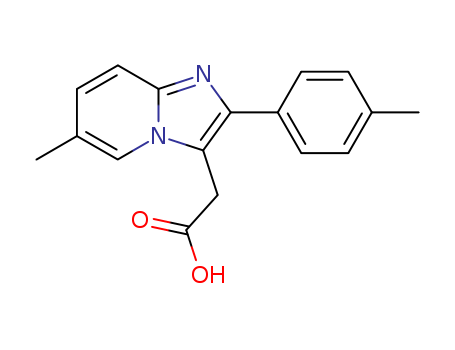
Zolpidic acid
CAS:189005-44-5
-
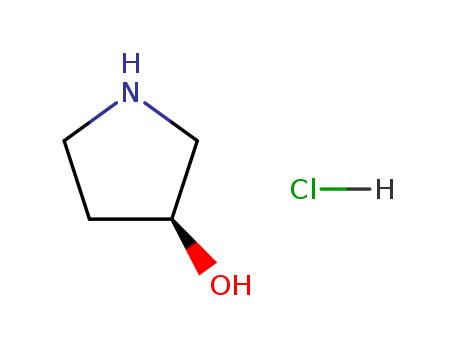
(S)-3-Hydroxypyrrolidine hydrochloride
CAS:122536-94-1