188425-85-6
- Product Name:BOSCALID
- Molecular Formula:C18H12Cl2N2O
- Purity:99%
- Molecular Weight:343.212
Product Details
pd_meltingpoint:142.8 to 143.8oC
Appearance:white powder
Export Top Purity BOSCALID 188425-85-6 In Stock
- Molecular Formula:C18H12Cl2N2O
- Molecular Weight:343.212
- Appearance/Colour:white powder
- Vapor Pressure:3.28E-08mmHg at 25°C
- Melting Point:142.8 to 143.8oC
- Refractive Index:1.666
- Boiling Point:447.728 °C at 760 mmHg
- PKA:10.75±0.70(Predicted)
- Flash Point:224.578 °C
- PSA:41.99000
- Density:1.369 g/cm3
- LogP:5.38070
Boscalid(Cas 188425-85-6) Usage
|
Fungicide |
Boscalid is a kind of nicotinamide germicide first successfully developed by BASF of Germany. It has a broad spectrum of bactericidal activity and has a preventive effect, being active against almost all types of fungal diseases. It has excellent effects on the control of powdery mildew, gray mold, root rot disease, sclerotinia and various kinds of rot diseases and is not easy to produce cross-resistance. It is also effective against the resistant bacteria to other agents. It is mainly used for the prevention and control of diseases associated with rape, grapes, fruit trees, vegetables and field crops. The results have showed that Boscalid had a significant effect on the treatment of Sclerotinia sclerotiorum with both the disease incidence control effect and the disease control index being higher than 80%, which was better than any of the other agents currently popularized. It has a significantly higher control efficacy than carbendazim. Apply 50% boscalid water solution dispersible granules to control rape sclerotia disease with a dose of 24 to 36 grams per acre medication per general year. In severe years, apply 36 to 48 grams per acre for medication. |
|
Toxicity |
(1) Mammalian toxicity: rat acute oral LD50> 5,000 mg/kg; Rat acute pertacuneous LD50 > 2,000 mg/kg, being non-irritating to skin and eyes of rabbits without sensitization to guinea pig skin. Rats inhaled LC50 (4 h)> 6.7 mg/L. NOEL: Rats, approximately 5 mg/kg (b. w.); Chronic NOAEL: 21.8 mg/kg (b. w.). [2003]; ADI/RfD (JMPR) 0.04 mg/kg (b.w.) [2006]; (EC) 0.04 mg/kg (b.w.) [2008]; cRfD 0.218 mg/kg (b.w.) [2003], (FSC) 0.044 mg/kg (b.w.) [2006]; other: no mutagenicity (Ames test, mouse), teratogenicity (rat, rabbit) and carcinogenic effects (dog, rat, mice); no adverse effects on reproduction (rat). (2) Ecotoxicity: Birds: Quail LD50> 2,000 mg/kg (b.w.). Fish: Rainbow trout LC50 (96 h) was 2.7 mg/L. Daphnia: EC50 (48 h) was 5.33 mg/L. The algae: Pseudokirchneriella subcapitata ErC50 (96 h) was 3.75 mg/L. Other aquatic organisms: Chironomus riparius NOEC 2.0 mg/L. Bee: NOEC (oral) is 166 μg /bee, (contact) is 200 μg/bee. Earthworm: Eisenia foetida LC50> 1,000 mg/kg (dry soil). (3) Environmental toxicity: animal: biphenyl ring is first subject to hydroxylation, followed by glucosylation and sulfation reaction. Boscalid is rapidly and extensively subject to metabolism in the body and excreted rapidly through the feces. Plants: biphenyl and pyridine ring are subject to hydroxylation and further ring-opening reaction. However, the parent compound, which has not been structurally altered, remains to be a major part of the residue. Soil/environment: it has moderate degradation action in the soil with the soil DT50 being 108 d ~> 1 a (laboratory, aerobic conditions, 20 ℃); the DT50 of the field is about 28d~200d. It has a excellent degradation property in natural water/sediment systems. |
|
Mechanism |
Boscalid is a kind of mitochondrion respiration inhibitor, being the inhibitor of the succinate dehydrogenase (SDHI) that acts by inhibiting succinate coenzyme Q reductase (also known as complex II) on the mitochondrial electron transport chain, with its mechanism of action being similar as that of other kinds of amide and benzamide fungicides. It has effects on the entire growth period of the pathogen, especially having a strong inhibitory effect against the spore germination. It also has excellent prophylactic effects and excellent intra-leaf permeability. Boscalid is a foliar application germicide, which can penetrate vertically and be transmitted to the top of the plant leaves. It has excellent preventive effect and has certain therapeutic effect. It can also inhibit the spore germination, germ tube elongation and attachment formation, and is effective in all other growth stages of the fungus, exhibiting excellent resistance to rain erosion and persistence. |
|
Preparation |
1.? Take o-chloronitrobenzene as raw material, first have it react with chlorobenzene boronic acid to undergo Suziki reaction, followed by reduction, and finally condensation with 2-chloronicotinyl chloride to obtain Boscalid crude product. 2. Take o-iodine aniline as raw material, first have reaction with 2-chloronicotinyl chloride, followed by Suzuki reaction with chlorobenzene boric acid to obtain the finished product. |
|
Patents |
On November 7, 2012, the patent on the European patent expired. On November 9, 2012, its patents in the United States expire. November 9, 2012, the administrative protection period of the compound in China expires. In 2003, Boscalid was registered in the United States and obtained a 10-year registration of data protection. In August 1, 2008, Boscalid was listed in the Appendix 1 of the EU pesticide registration directive (91/414) with its registration information being protected to until July 31, 2018. The patent and administrative protection in Europe and the United States and China will soon expire, but the EU data protein is far lagged behind from its patent protection. Non-patented products manufacturers, if needs to enter the European and American markets, must prepare a complete set of registration information, or consult the data owner. Alternatively, they can enter into the markets until the information protein is expired. However, boscalid will soon become a focus of those non-patented manufactures in China, which will lead to the escalation of the Chinese market completion of boscalid. |
|
Definition |
ChEBI: A pyridinecarboxamide obtained by formal condensation of the carboxy group of 2-chloronicotinic acid with the amino group of 4'-chlorobiphenyl-2-amine. A fungicide active against a broad range of fungal pathogens including Botrytis spp., |
InChI:InChI=1/C18H12Cl2N2O/c19-13-9-7-12(8-10-13)14-4-1-2-6-16(14)22-18(23)15-5-3-11-21-17(15)20/h1-11H,(H,22,23)
188425-85-6 Relevant articles
Synthesis and process optimization of Boscalid by catalyst Pd-PEPPSI-IPrDtBu-An
Xu, Jian,Lan, Xiao-Bing,Xia, Lin-Jian,Yang, Yi,Cao, Gao
, p. 247 - 256 (2021/05/06)
The purpose of this research was to redu...
Preparation method of acrylamido
-
Paragraph 0118; 0156; 0159-0160; 0162, (2022/01/04)
The present invention relates to a metho...
Pd-Catalysed Suzuki-Miyaura cross-coupling of aryl chlorides at low catalyst loadings in water for the synthesis of industrially important fungicides
Goetz, Roland,Hashmi, A. Stephen K.,Orecchia, Patrizio,Petkova, Desislava Slavcheva,Rominger, Frank,Schaub, Thomas
supporting information, p. 8169 - 8180 (2021/11/01)
The Suzuki-Miyaura coupling reaction of ...
A Sustainable 1-Pot, 3-Step Synthesis of Boscalid Using Part per Million Level Pd Catalysis in Water
Takale, Balaram S.,Thakore, Ruchita R.,Mallarapu, Rushil,Gallou, Fabrice,Lipshutz, Bruce H.
, p. 101 - 105 (2019/12/30)
Boscalid is an active ingredient in seve...
188425-85-6 Process route
-
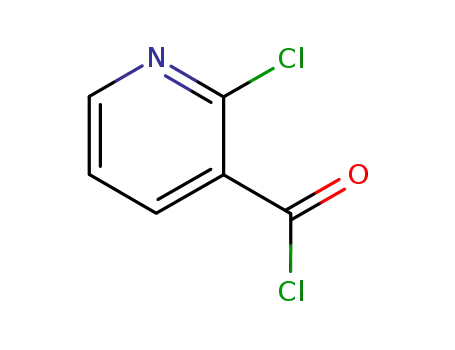
-
49609-84-9
2-Chloronicotinoyl chloride

-
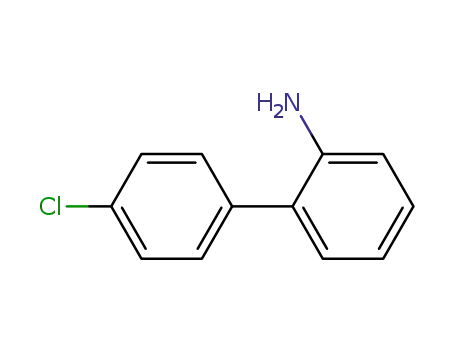
-
1204-44-0
2-(4-chlorophenyl)aniline

-
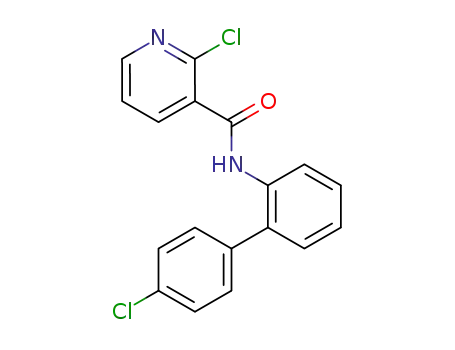
-
188425-85-6
boscalid
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
water;
at 20 - 45 ℃;
for 16h;
Reagent/catalyst;
|
97% |
|
In
isopropyl alcohol;
at 40 - 65 ℃;
for 4h;
Solvent;
Temperature;
|
95% |
|
With
potassium carbonate;
In
dichloromethane;
at 20 ℃;
for 1h;
Reagent/catalyst;
|
95.04% |
|
With
triethylamine;
In
tetrahydrofuran;
at 25 ℃;
for 1h;
|
94% |
|
With
sodium carbonate;
In
dichloromethane;
at 0 - 40 ℃;
for 0.018055h;
|
94% |
|
With
triethylamine;
In
tetrahydrofuran;
at 25 ℃;
for 1h;
|
93% |
|
In
5,5-dimethyl-1,3-cyclohexadiene;
for 5h;
Solvent;
Reflux;
|
92.3% |
|
With
sodium carbonate; aniline;
In
water;
at 75 - 90 ℃;
for 1h;
Concentration;
|
91% |
|
at 50 ℃;
|
91.7% |
|
With
triethylamine;
In
dichloromethane;
at 25 ℃;
for 0.166667h;
|
89% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 6h;
Reflux;
|
87% |
|
With
triethylamine;
In
dichloromethane;
for 6h;
Reflux;
|
87% |
|
With
triethylamine;
In
dichloromethane;
for 6h;
Reflux;
|
87% |
|
With
triethylamine;
In
dichloromethane;
at 25 ℃;
for 12h;
|
83% |
|
With
dmap; triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 13h;
|
81% |
|
2-(4-chlorophenyl)aniline;
With
triethylamine;
In
water; ethyl acetate;
at 20 ℃;
for 0.166667h;
2-Chloronicotinoyl chloride;
In
water; ethyl acetate;
at 60 ℃;
for 18h;
|
75% |
|
With
sodium carbonate;
In
dichloromethane;
at 20 ℃;
for 3h;
|
60% |
|
With
triethylamine;
In
tetrahydrofuran;
at 25 ℃;
for 1h;
|
270 mg |
|
With
pyridine;
at 100 ℃;
for 12h;
Inert atmosphere;
|
|
|
With
dmap; triethylamine;
In
dichloromethane;
at 20 ℃;
for 14h;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
97 mg |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 3h;
Time;
|
|
|
With
triethylamine;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 30 - 95 ℃;
Reagent/catalyst;
Solvent;
Temperature;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
at 23 ℃;
for 1h;
Inert atmosphere;
Sealed tube;
|
181 mg |
|
In
tetrahydrofuran; water;
at 45 ℃;
for 8h;
|
-

-
49609-84-9
2-Chloronicotinoyl chloride

-
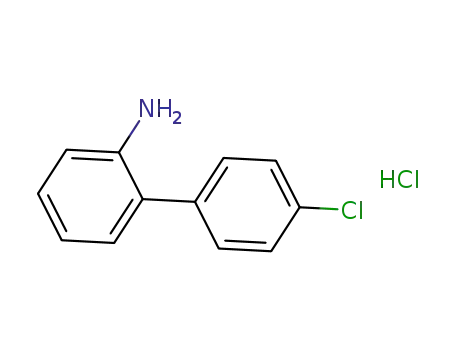
-
4'-chloro-2-acetamidobiphenyl hydrochloride

-

-
188425-85-6
boscalid
| Conditions | Yield |
|---|---|
|
4'-chloro-2-acetamidobiphenyl hydrochloride;
With
sodium carbonate; triethylamine;
In
dichloromethane;
at 35 ℃;
for 0.5h;
2-Chloronicotinoyl chloride;
In
dichloromethane;
for 8h;
Solvent;
|
96.3% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 3h;
|
92.1% |
188425-85-6 Upstream products
-
2942-59-8
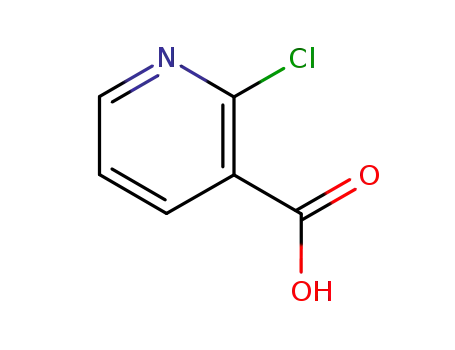
2-chloronicotinic acid
-
1204-44-0

2-(4-chlorophenyl)aniline
-
1101170-92-6
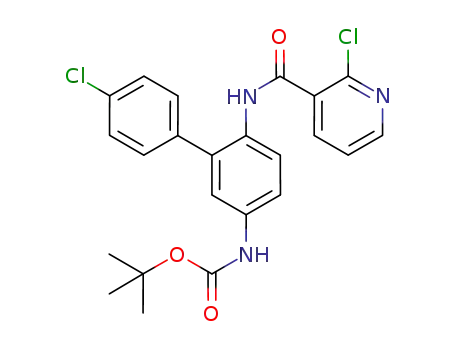
tert-butyl {4'-chloro-6-[(2-chloropyridine-3-carbonyl)amino]biphen-3-yl}carbamate
-
49609-84-9

2-Chloronicotinoyl chloride
188425-85-6 Downstream products
-
1304051-01-1
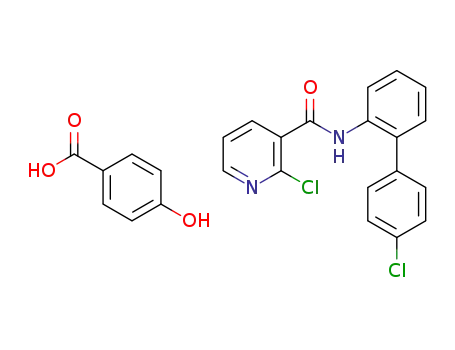
C7H6O3*C18H12Cl2N2O
Relevant Products
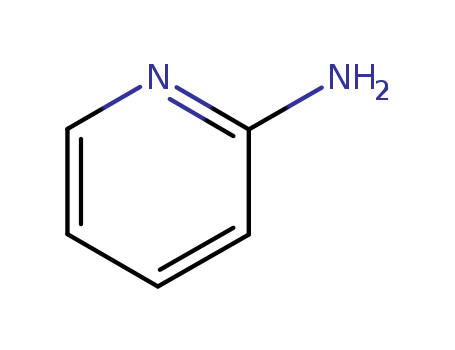
-
2-Aminopyridine
CAS:504-29-0
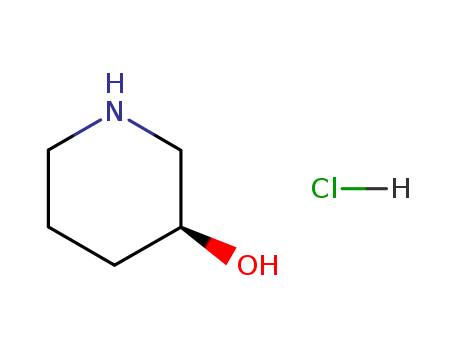
-
(S)-3-Hydroxypiperidine hydrochloride
CAS:475058-41-4