504-29-0
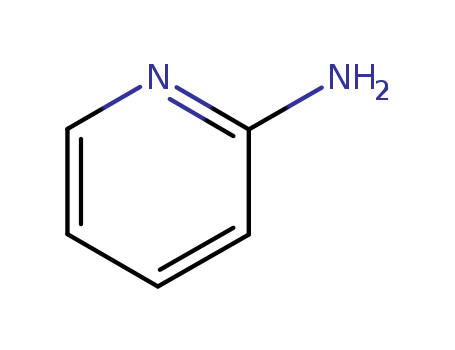
- Product Name:2-Aminopyridine
- Molecular Formula:C5H6N2
- Purity:99%
- Molecular Weight:94.116
Product Details
pd_meltingpoint:59 °C
Appearance:cream to light yellow-beige crystalline powder
Factory Supply Industrial Grade 2-Aminopyridine 504-29-0 with Best Price
- Molecular Formula:C5H6N2
- Molecular Weight:94.116
- Appearance/Colour:cream to light yellow-beige crystalline powder
- Vapor Pressure:0.191mmHg at 25°C
- Melting Point:59 °C
- Refractive Index:1.5560 (estimate)
- Boiling Point:210.6 °C at 760 mmHg
- PKA:6.82(at 20℃)
- Flash Point:98.7 °C
- PSA:38.91000
- Density:1.107g/cm3
- LogP:1.24500
2-Aminopyridine(Cas 504-29-0) Usage
|
Preparation |
2-Aminopyridine is manufactured using the reaction of pyridine with sodium amide (Chichibabin amination). It is obtained in high yield after the hydrolysis of the intermediate salt (Merck, 2001; Shimizu et al., 1993). |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 72, p. 4554, 2007 DOI: 10.1021/jo070189yTetrahedron Letters, 11, p. 3901, 1970 |
|
Air & Water Reactions |
Decomposes in air. Soluble in water. |
|
Reactivity Profile |
2-Aminopyridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. Reacts with oxidizing agents . |
|
Hazard |
Toxic. |
|
Health Hazard |
2-Aminopyridine causes central nervous system effects. |
|
Fire Hazard |
2-Aminopyridine is combustible. |
|
Safety Profile |
Poison by ingestion, inhalation, subcutaneous, intravenous, and intraperitoneal routes. Toxic effects resemble strychnine poisoning. Human systemic effects by inhalation: somnolence, convulsions, and antipsychotic effects. Human central nervous system effects by inhalation. When heated to decomposition it emits highly toxic fumes of NOx,. |
|
Potential Exposure |
2-Aminopyridine is used in the manufacture of pharmaceuticals; especially antihistamines. |
|
Carcinogenicity |
The LD50 in mice by intraperitoneal injection was 35 mg/kg; lethal doses in animals also produced excitement, tremors, convulsions and tetany.1 Fatal doses were readily absorbed through the skin. A 0.2 M aqueous solution dropped in a rabbit’s eye was only mildly irritating. 2-Aminopyridine was not mutagenic in a variety of Salmonella tester strains with or without metabolic activation. |
|
Environmental fate |
Soil. When radio-labeled 4-aminopyridine was incubated in moist soils (50%) under aerobic conditions at 30 °C, the amount of 14CO2 released from an acidic loam (pH 4.1) and an alkaline, loamy sand (pH 7.8) was 0.4 and 50%, respectively (Starr and Cunningham, 1975). Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and Lewis, 1987). |
|
Shipping |
UN2671 Aminopyridines, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
|
Purification Methods |
It crystallises from *benzene/pet ether (b 40-60o) or CHCl3 /pet ether. [Beilstein 22/8 V 280.] |
|
Waste Disposal |
Incineration with nitrogen oxides removal from effluent gas. |
|
Application |
2-Aminopyridine has also been used to derivatize sialyloligosaccharides for detection in FAB-MS. It can also be used:As a reactant in the synthesis of 3-aroylimidazopyridines from chalcones by aerobic oxidative amidation using copper acetate catalyst.In the synthesis of crystalline Cu(II) complex, di-μ-(2-aminopyridine(N,N′))-bis[(2,6 pyridinedicarboxylate)aquacopper(II)] tetrahydrate using 2,6-pyridinedicarboxylic acid and Cu(CH3COO)2.H2O.As an imprinting molecule for the preparation of poly(methacrylic acid–ethylene glycol dimethacrylate) polymer. It is packed in micro-column for selective solid phase extraction of 2-aminopyridine.As a reactant in the synthesis of 2-aryl-3-(pyridin-2-yl)-1,3-thiazolidin-4-ones in the presence of Lewis acid catalysts.As a reactant in the synthesis of 2-(2-aminopyridinium)acetyl starch with antioxidant property. |
|
General Description |
2-aminopyridine appears as white powder or crystals or light brown solid. It is soluble in water and alcohol. It is toxic by ingestion and by inhalation of the dust. It is used to make pharmaceuticals and dyes. (NTP, 1992) |
InChI:InChI=1/C5H6N2/c6-5-3-1-2-4-7-5/h1-4H,(H2,6,7)/p+1
504-29-0 Relevant articles
A novel approach towards chemoselective reduction of nitro to amine
Dasgupta, Hridoydip Ranjan,Mukherjee, Suvodip,Ghosh, Pranab
, (2019)
Chemo selective reduction of a wide rang...
-
Adams,Pachter
, p. 4906 (1952)
-
One-Pot Fabrication of Pd Nanoparticles?Covalent-Organic-Framework-Derived Hollow Polyamine Spheres as a Synergistic Catalyst for Tandem Catalysis
Yang, Xinyi,He, Yajun,Li, Liuyi,Shen, Jinni,Huang, Jianhui,Li, Lingyun,Zhuang, Zanyong,Bi, Jinhong,Yu, Yan
, p. 1864 - 1870 (2020)
Facile fabrication of nanocatalysts cons...
N,N-Chelate nickel(II) complexes bearing Schiff base ligands as efficient hydrogenation catalysts for amine synthesis
Xu, Mengyin,Wang, Yang,Zhou, Yifeng,Yao, Zi-Jian
, (2021/12/09)
Five N, N-chelate nickel (II) complexes ...
Dual Reactivity of 1,2,3,4-Tetrazole: Manganese-Catalyzed Click Reaction and Denitrogenative Annulation
Chattopadhyay, Buddhadeb,Das, Sandip Kumar,Khatua, Hillol,Roy, Satyajit
, p. 304 - 312 (2020/10/29)
A general catalytic method using a Mn-po...
Development and Application of Efficient Ag-based Hydrogenation Catalysts Prepared from Rice Husk Waste
Unglaube, Felix,Kreyenschulte, Carsten Robert,Mejía, Esteban
, p. 2583 - 2591 (2021/04/09)
The development of strategies for the su...
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications
Zhao, Lixing,Hu, Chenyang,Cong, Xuefeng,Deng, Gongda,Liu, Liu Leo,Luo, Meiming,Zeng, Xiaoming
supporting information, p. 1618 - 1629 (2021/01/25)
Transition metal catalysis that utilizes...
504-29-0 Process route
-
-
25142-28-3
2-phenyl-1H-imidazo<1,2-a>pyridinium-3-olate

-
-
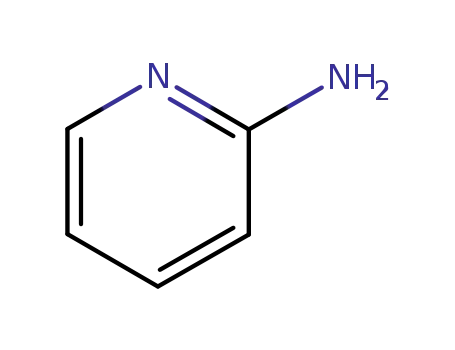
504-29-0
2-aminopyridine

-
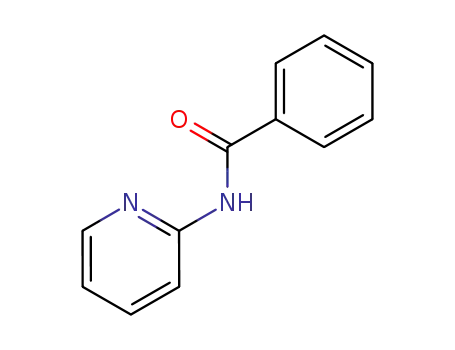
-
4589-12-2
N-(pyrid-2-yl)benzamide

-
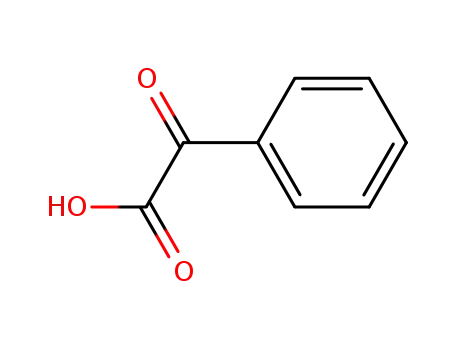
-
611-73-4
Benzoylformic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
|
-
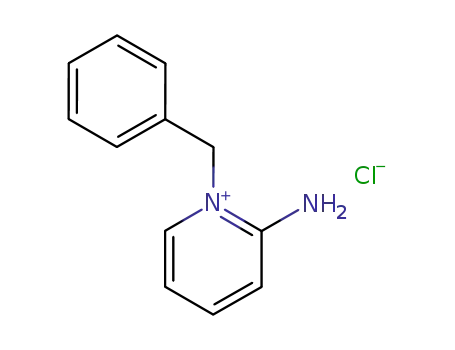
-
141206-63-5
2-amino-1-benzylpyridinium chloride

-

-
504-29-0
2-aminopyridine

-
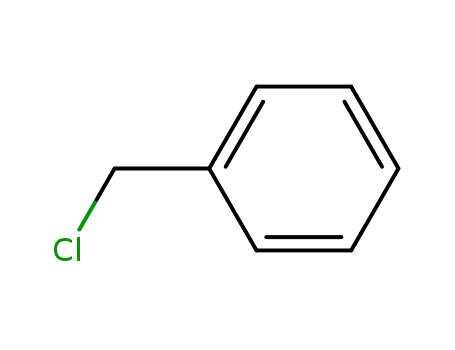
-
100-44-7
benzyl chloride

-
-
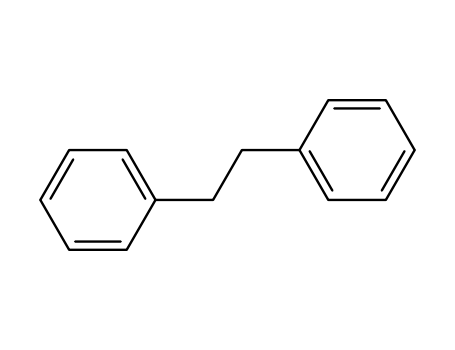
103-29-7
1,1'-(1,2-ethanediyl)bisbenzene

-
-
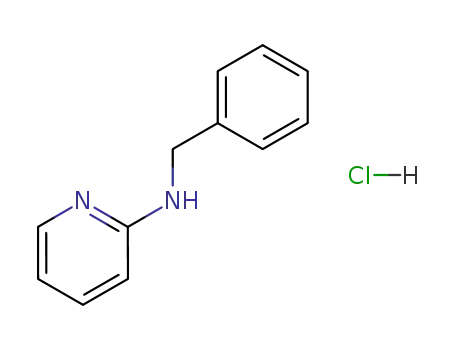
67465-04-7
2-benzylaminopyridine hydrochloride

-
-
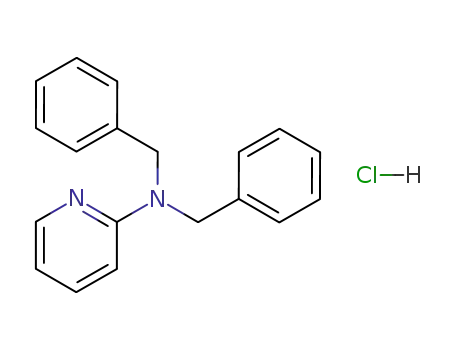
2-(N,N-dibenzylamino)pyridine hydrochloride

-
-
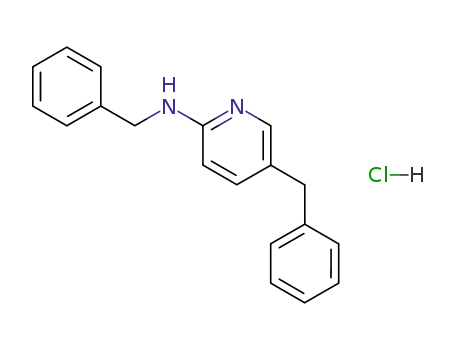
2-benzylamino-5-benzylpyridine hydrochloride
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
at 235 - 250 ℃;
for 2h;
Product distribution;
Mechanism;
variation of time;
|
504-29-0 Upstream products
-
110-86-1
pyridine
-
109-09-1
2-chloropyridine
-
109-04-6
2-bromo-pyridine
-
31888-72-9
pyridine-2-carbohydroxamic acid
504-29-0 Downstream products
-
343269-67-0
1-(2-hydroxy-ethyl)-1H-pyridin-2-one-imine
-
71862-98-1
N,N'-di-[2]pyridyl-benzene-1,4-disulfonamide
-
1657-28-9
3-oxo-N-(pyrid-2-yl)butyramide
-
1202-34-2
di(pyridin-2-yl)amine
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
11-alpha-Hydroxycarvenone
CAS:192569-17-8
-
BOSCALID
CAS:188425-85-6