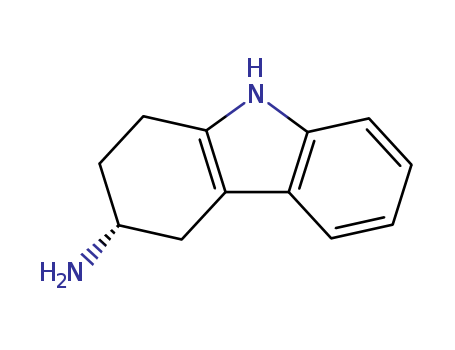
116650-33-0
- Product Name:(3R)-3-amino-1,2,3,4-tetrahydrocarbazole
- Molecular Formula:C12H14N2
- Purity:99%
- Molecular Weight:186.257
Product Details
Factory Sells Best Quality (3R)-3-amino-1,2,3,4-tetrahydrocarbazole 116650-33-0 with ISO standards
- Molecular Formula:C12H14N2
- Molecular Weight:186.257
- Refractive Index:1.677
- Boiling Point:362.512 °C at 760 mmHg
- PKA:17.54±0.40(Predicted)
- Flash Point:200.5 °C
- PSA:41.81000
- Density:1.192 g/cm3
- LogP:2.68420
(R)-3-Amino-1,2,3,4-tetrahydrocarbazole(Cas 116650-33-0) Usage
|
General Description |
(R)-3-Amino-1,2,3,4-tetrahydrocarbazole is a chemical compound that belongs to the class of tetrahydrocarbazole derivatives. It is an organic compound with the molecular formula C13H14N2 and a molecular weight of 198.27 g/mol. (R)-3-Amino-1,2,3,4-tetrahydrocarbazole has a chiral center, and its (R) enantiomer is the biologically active form. (R)-3-Amino-1,2,3,4-tetrahydrocarbazole has been studied for its potential medicinal properties, including its ability to act as a serotonin receptor agonist. It may have applications in pharmaceutical research for the development of new drugs targeting serotonin receptors and for studying the role of serotonin in physiological and pathological conditions. |
InChI:InChI=1/C12H14N2/c13-8-5-6-12-10(7-8)9-3-1-2-4-11(9)14-12/h1-4,8,14H,5-7,13H2/t8-/m1/s1
116650-33-0 Relevant articles
Microenvironmental effects on the solvent quenching rate in constrained tryptophan derivatives
Yu, Hong-Tao,Vela, Marco A.,Fronczek, Frank R.,McLaughlin, Mark L.,Barkley, Mary D.
, p. 348 - 357 (1995)
Solvent quenching is one of several envi...
Cutting short the asymmetric synthesis of the ramatroban precursor by employing ω-transaminases
Busto, Eduardo,Simon, Robert C.,Grischek, Barbara,Gotor-Fernandez, Vicente,Kroutil, Wolfgang
, p. 1937 - 1942 (2014)
Starting from an adequate ketone precurs...
Fluorescence Studies with Tryptophan Analogues: Excited State Interactions Involving the Side Chain Amino Group
Eftink, Maurice R.,Jia, Yiwei,Hu, Dana,Ghiron, Camillo, A.
, p. 5713 - 5723 (1995)
The fluorescence of a large set of trypt...
-
Dressler,Baum
, p. 102 (1961)
-
INDOLE AHR INHIBITORS AND USES THEREOF
-
, (2018/11/22)
The present invention provides compounds...
Synthesis method for (R)-3-amino-1,2,3,4-tetrahydrocarbazole
-
, (2016/10/08)
The invention discloses a synthesis meth...
Asymmetric chemoenzymatic synthesis of ramatroban using lipases and oxidoreductases
Busto, Eduardo,Gotor-Fernandez, Vicente,Gotor, Vicente
scheme or table, p. 4842 - 4848 (2012/07/31)
A chemoenzymatic asymmetric route for th...
116650-33-0 Process route
-
![1,4-dioxaspiro[4.5]decan-8-amine](/upload/2024/12/1dd9ce16-3515-480a-8e61-d520bc74bd99.png)
-
97096-16-7
1,4-dioxaspiro[4.5]decan-8-amine

-
-
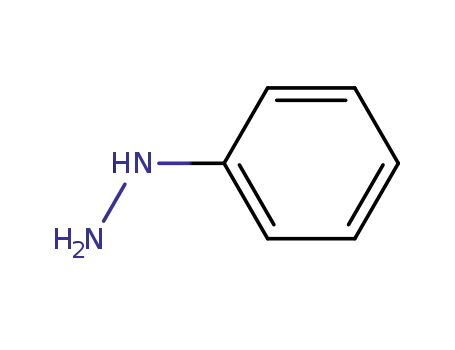
100-63-0
phenylhydrazine

-
-
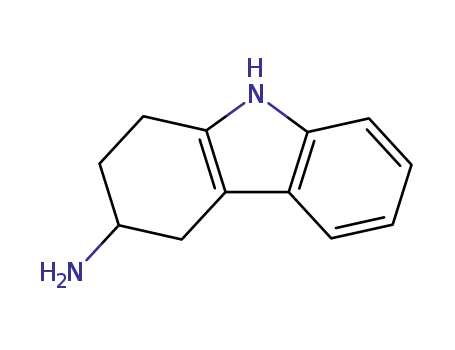
61894-99-3,116650-33-0,116650-34-1,134525-54-5
2,3,4,9-tetrahydro-1H-carbazol-3-yl-amine
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
water;
at 95 - 100 ℃;
|
50% |
-
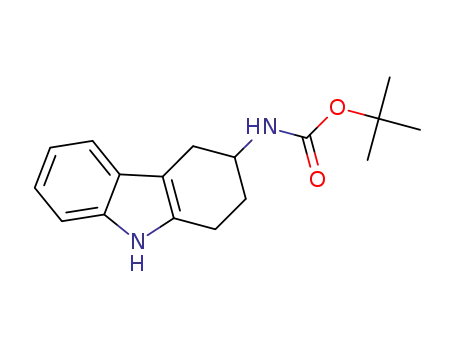
-
1392271-79-2
tert-butyl N-(2,3,4,9-tetrahydro-1H-carbazol-3-yl)carbamate

-

-
61894-99-3,116650-33-0,116650-34-1,134525-54-5
2,3,4,9-tetrahydro-1H-carbazol-3-yl-amine
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
dichloromethane;
at 28 ℃;
for 2h;
|
116650-33-0 Upstream products
-
51145-61-0
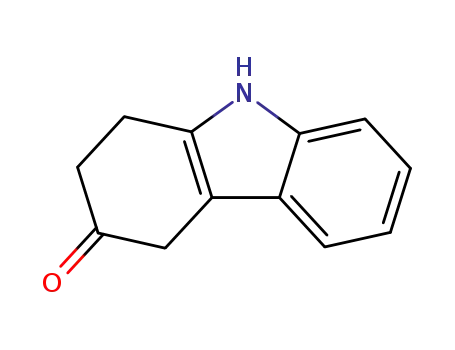
1,2,3,4-tetrahydrocarbazol-3-one
-
34154-31-9
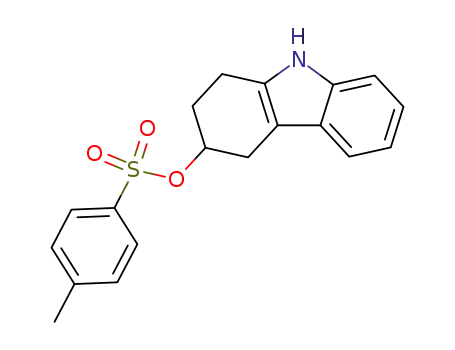
3-hydroxy-1,2,3,4-tetrahydrocarbazole p-toluenesulphonate ester
-
128432-38-2
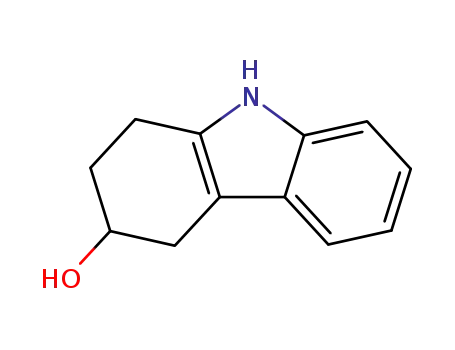
(+/-)-2,3,4,9-tetrahydro-1H-carbazol-3-ol
-
611-71-2
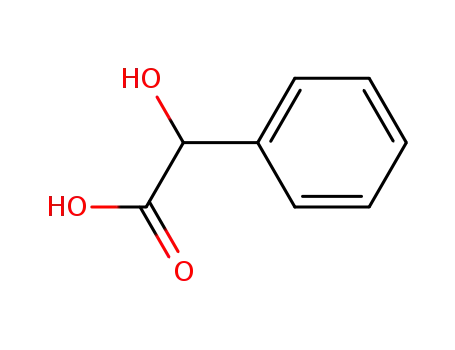
MANDELIC ACID
116650-33-0 Downstream products
-
60480-69-5
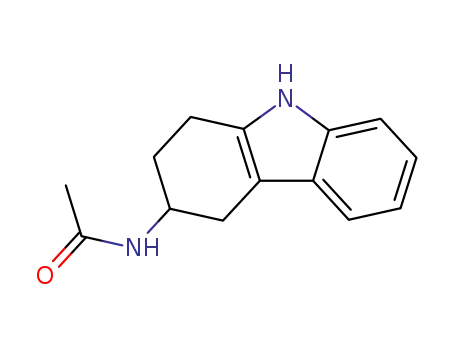
N-(2,3,4,9-tetrahydro-1H-carbazol-3-yl)acetamide
-
1374648-20-0
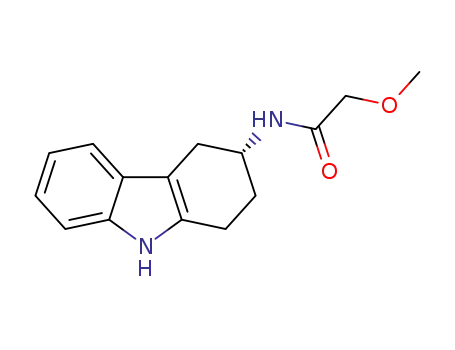
(R)-2-methoxy-N-(2,3,4,9-tetrahydro-1H-carbazol-3-yl)acetamide
-
116650-34-1
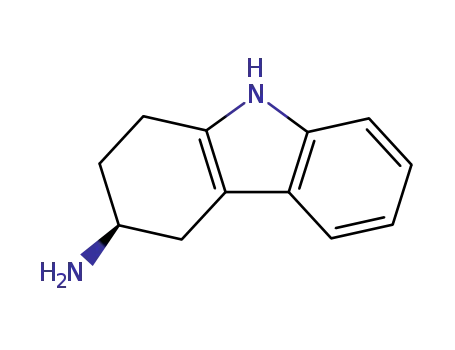
(3S)-2,3,4,9-tetrahydro-1H-carbazol-3-amine
-
116650-12-5
4-fluoro-N-(2,3,4,9-tetrahydro-1H-carbazol-3-yl)benzenesulfonamide
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
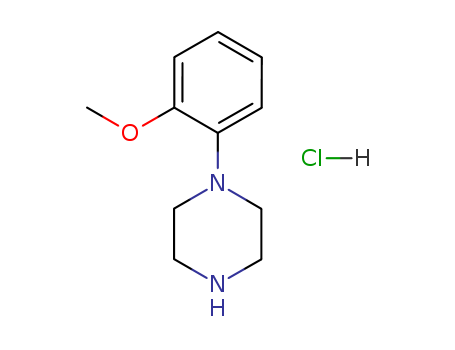
1-(2-Methoxyphenyl)piperazine hydrochloride
CAS:5464-78-8
-
Dodecyltrimethylammonium chloride
CAS:112-00-5