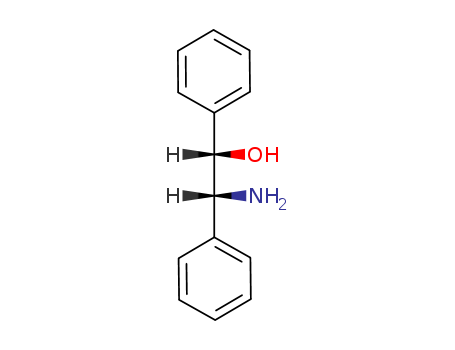
23364-44-5
- Product Name:(1S,2R)-2-Amino-1,2-diphenylethanol
- Molecular Formula:C14H15NO
- Purity:99%
- Molecular Weight:213.279
Product Details
pd_meltingpoint:142-144 ºC
Appearance:white to light yellow crystal powde
High Quality Chinese Manufacturer supply 23364-44-5 (1S,2R)-2-Amino-1,2-diphenylethanol
- Molecular Formula:C14H15NO
- Molecular Weight:213.279
- Appearance/Colour:white to light yellow crystal powde
- Vapor Pressure:2.88E-06mmHg at 25°C
- Melting Point:142-144 ºC
- Refractive Index:7 ° (C=0.6, EtOH)
- Boiling Point:374.3 ºC at 760 mmHg
- PKA:11.70±0.45(Predicted)
- Flash Point:180.2 ºC
- PSA:46.25000
- Density:1.148 g/cm3
- LogP:3.12030
(1S,2R)-2-Amino-1,2-diphenylethanol(Cas 23364-44-5) Usage
|
Reaction |
Ligand used to make chiral oxaborolidines for the enantioselective alkynylation of aldehydes Ligand used in organoindium reagents for asymmetric Barbier-type allylations Ligand used in organoindium reagents for asymmetric Barbier-type propargylations |
InChI:InChI=1/C14H15NO/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14,16H,15H2/p+1/t13-,14+/m1/s1
23364-44-5 Relevant articles
Site-Specific C(sp3)–H Aminations of Imidates and Amidines Enabled by Covalently Tethered Distonic Radical Anions
Fang, Yuanding,Fu, Kang,Shi, Lei,Zhao, Rong,Zhou, Jia
supporting information, p. 20682 - 20690 (2020/09/07)
The utilization of N-centered radicals t...
Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides
Marcyk, Paul T.,Jefferies, Latisha R.,AbuSalim, Deyaa I.,Pink, Maren,Baik, Mu-Hyun,Cook, Silas P.
supporting information, p. 1727 - 1731 (2019/01/21)
The direct, catalytic substitution of un...
Large-scale preparation of key building blocks for the manufacture of fully synthetic macrolide antibiotics
Hogan, Philip C.,Chen, Chi-Li,Mulvihill, Kristen M.,Lawrence, Jonathan F.,Moorhead, Eric,Rickmeier, Jens,Myers, Andrew G.
, p. 318 - 325 (2018/03/21)
Key building blocks for the production o...
Chiral morphine quinoline compound preparation method and chiral amino acid preparation method of compound
-
Paragraph 0105; 0107; 0108; 0110, (2017/12/13)
The invention provides chiral morpholine...
23364-44-5 Process route
-
-
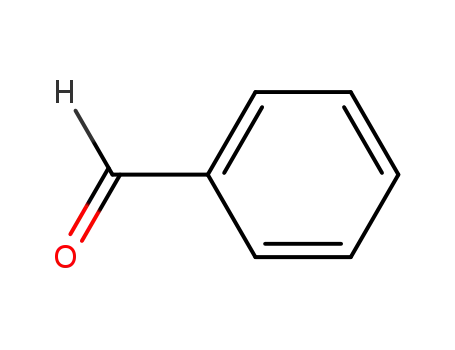
100-52-7
benzaldehyde

-
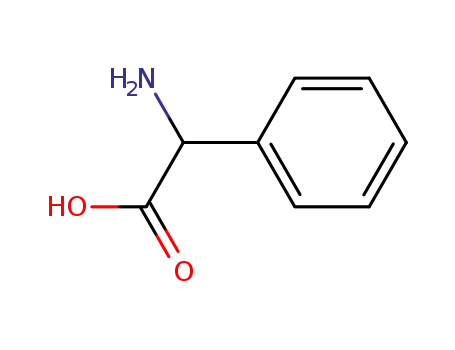
-
2835-06-5
phenylglycin

-
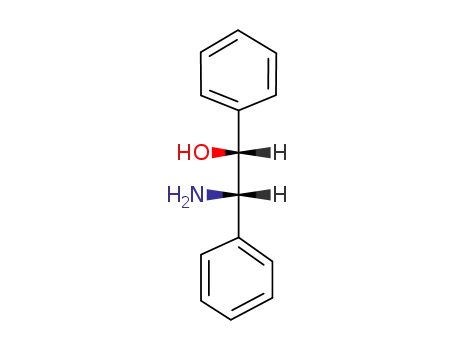
-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
erythro-2-amino-1,2-diphenylethanol

-
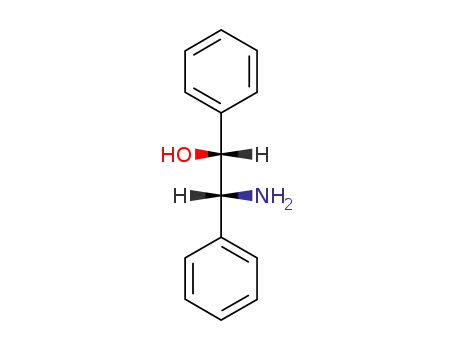
-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
threo-1,2-diphenyl-2-aminoethan-1-ol

-
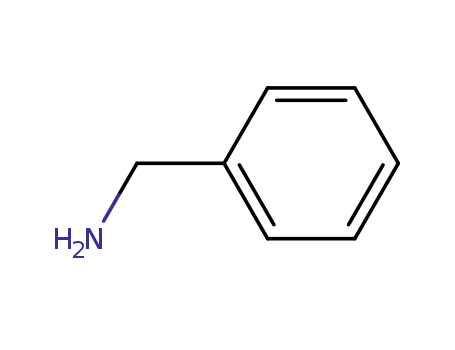
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
|
-

-
100-52-7
benzaldehyde

-

-
2835-06-5
phenylglycin

-

-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
erythro-2-amino-1,2-diphenylethanol

-

-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
threo-1,2-diphenyl-2-aminoethan-1-ol

-
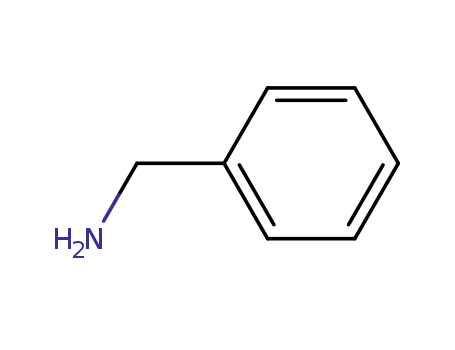
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
|
23364-44-5 Upstream products
-
64-17-5
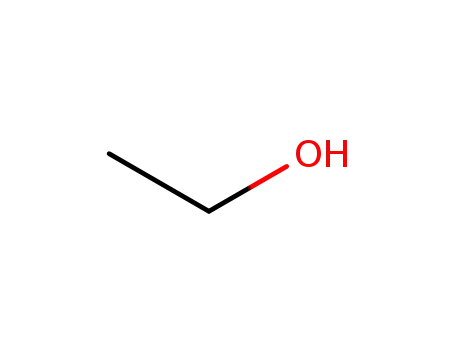
ethanol
-
87-69-4
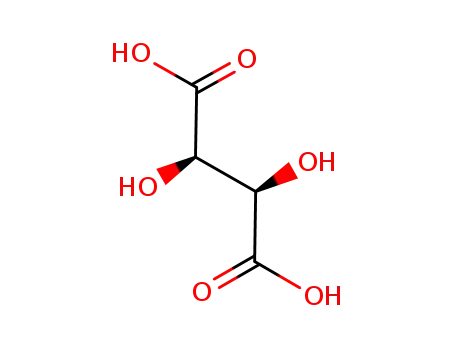
L-Tartaric acid
-
530-36-9

threo-1,2-diphenyl-2-aminoethan-1-ol
-
572-45-2
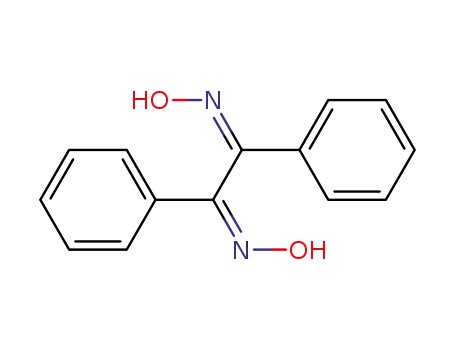
benzil dioxime
23364-44-5 Downstream products
-
92552-75-5
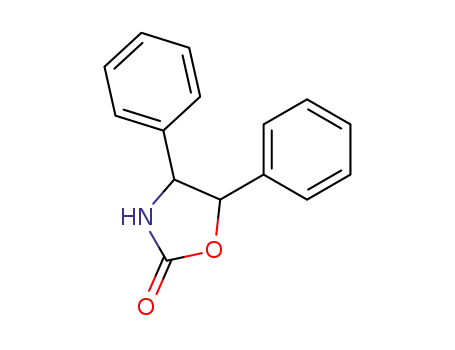
4,5-diphenyl-2-oxazolidinone
-
6275-45-2
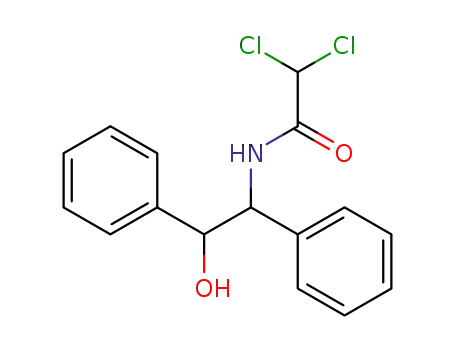
dichloro-acetic acid-(α'-hydroxy-bibenzyl-α-ylamide)
-
530-36-9
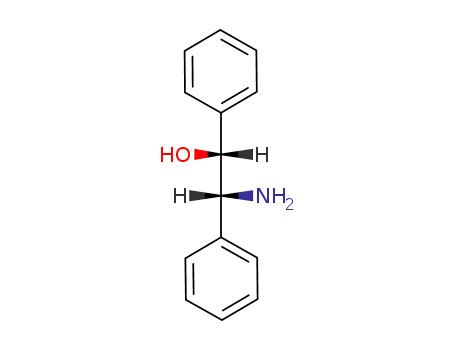
(1R,2R)-2-amino-1,2-diphenylethane-1-ol
-
492-70-6
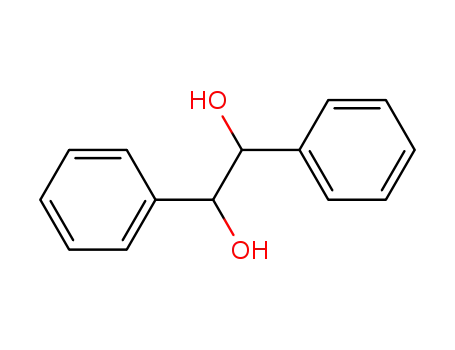
1,2-diphenyl-1,2-ethanediol
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
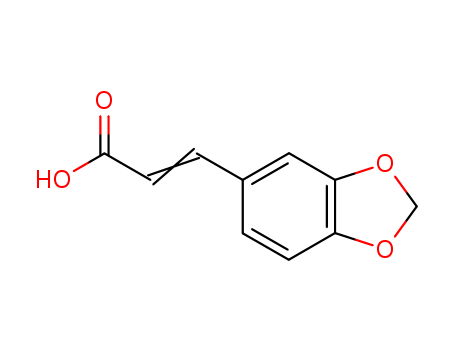
3,4-(Methylenedioxy)cinnamic acid
CAS:2373-80-0
-
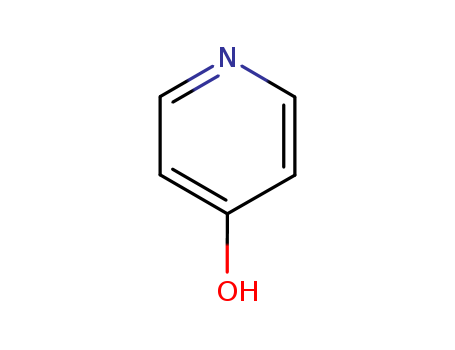
4-Hydroxypyridine
CAS:626-64-2