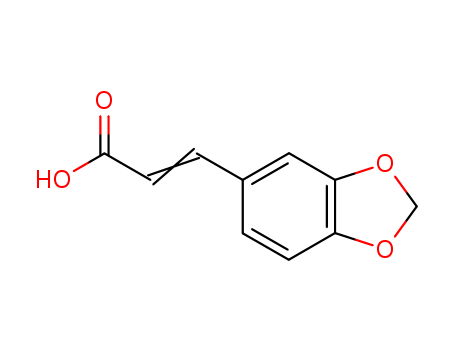
2373-80-0
- Product Name:3,4-(Methylenedioxy)cinnamic acid
- Molecular Formula:C10H8O4
- Purity:99%
- Molecular Weight:192.171
Product Details
pd_meltingpoint:242-244 °C (dec.)(lit.)
Appearance:white to light yellow granular powder
Factory Sells Best Quality 3,4-(Methylenedioxy)cinnamic acid 2373-80-0 with ISO standards
- Molecular Formula:C10H8O4
- Molecular Weight:192.171
- Appearance/Colour:white to light yellow granular powder
- Vapor Pressure:7.39E-06mmHg at 25°C
- Melting Point:242-244 °C (dec.)(lit.)
- Refractive Index:1.4440 (estimate)
- Boiling Point:361.5oC at 760 mmHg
- PKA:4.37±0.10(Predicted)
- Flash Point:148.9oC
- PSA:55.76000
- Density:1.41 g/cm3
- LogP:1.51310
3,4-(Methylenedioxy)cinnamic acid(Cas 2373-80-0) Usage
|
Purification Methods |
Crystallise the acid from glacial AcOH, EtOH (m 247o) or aqueous EtOH (m 240-242o), and it has m 242o after sublimation. [Beilstein 19 H 278, 19 II 299, 19 III/IV 3548.] |
|
General Description |
3,4-(Methylenedioxy)cinnamic acid is an inhibitor of the phenylpropanoid enzyme 4-hydroxycinnamoyl-CoA ligase. It undergoes electron transfer reaction with trichloromethylperoxyl radical and reaction has been studied by pulse radiolysis. |
InChI:InChI=1/C10H8O4/c11-10(12)4-2-7-1-3-8-9(5-7)14-6-13-8/h1-5H,6H2,(H,11,12)/p-1/b4-2+
2373-80-0 Relevant articles
Synthesis, characterization, antidepressant and antioxidant activity of novel piperamides bearing piperidine and piperazine analogues
Prashanth,Revanasiddappa, Hosakere D.,Lokanatha Rai,Veeresh
, p. 7065 - 7070 (2012)
A series of piperamide derivatives (8a-j...
Studies on the constituents of chloranthus spp. I. The structures of two new amides from Chloranthus serratus and the isolation of isofraxidin from C. japonicus
Takemoto,Uchida,Koike,et al.
, p. 1161 - 1163 (1975)
-
4-Alkyliden-azetidinones modified with plant derived polyphenols: Antibacterial and antioxidant properties
Giacomini, Daria,Musumeci, Rosario,Galletti, Paola,Martelli, Giulia,Assennato, Lorenzo,Sacchetti, Gianni,Guerrini, Alessandra,Calaresu, Enrico,Martinelli, Marianna,Cocuzza, Clementina
, p. 604 - 614 (2017)
Antimicrobial resistance is one of the m...
Dual Nickel/Ruthenium Strategy for Photoinduced Decarboxylative Cross-Coupling of α,β-Unsaturated Carboxylic Acids with Cycloketone Oxime Esters
Gao, Ang,Jiang, Run-Chuang,Liu, Chuang-Chuang,Liu, Qi-Le,Lu, Xiao-Yu,Xia, Ze-Jie
supporting information, p. 8829 - 8842 (2021/06/30)
Herein, a dual nickel/ruthenium strategy...
Simplified Derivatives of Dibenzylbutyrolactone Lignans from Hydrocotyle bonariensis as Antitrypanosomal Candidates
Souza, Dalete Christine S.,Costa-Silva, Thais A.,Morais, Thiago R.,Brito, Juliana R.,Ferreira, Edgard A.,Antar, Guilherme M.,Sartorelli, Patricia,Tempone, Andre G.,Lago, Jo?o Henrique G.
, (2021/10/01)
The search for the pharmacophore of a bi...
Quorum sensing and nf-κb inhibition of synthetic coumaperine derivatives from piper nigrum
Baruch, Yifat,Gopas, Jacob,Kadosh, Yael,Kumar, Rajendran Saravana,Kushmaro, Ariel,Muthuraman, Subramani,Yaniv, Karin
supporting information, (2021/05/28)
Bacterial communication, termed Quorum S...
Piperine derivative as well as preparation method and application thereof
-
Paragraph 0255; 0257-0259, (2020/05/08)
The invention provides a piperine deriva...
2373-80-0 Process route
-
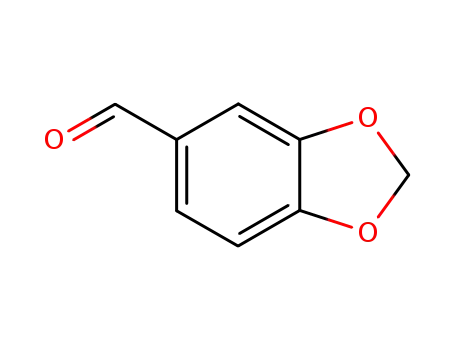
-
120-57-0,30024-74-9
piperonal

-
-
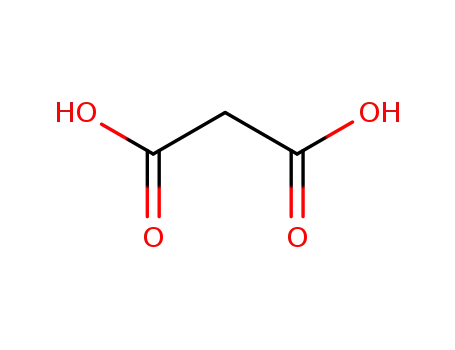
141-82-2
malonic acid

-
-
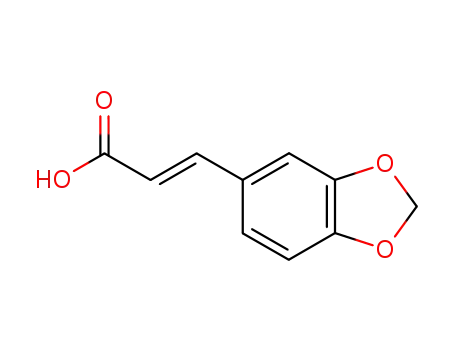
2373-80-0,38489-76-8
3,4-methylenedioxy-trans-cinnamic acid
| Conditions | Yield |
|---|---|
|
With
aluminum oxide; lithium chloride;
for 0.1h;
microwave irradiation;
|
98% |
|
With
piperidine; pyridine;
for 1h;
Reflux;
|
98% |
|
With
ammonium acetate;
for 0.0666667h;
Irradiation;
|
97% |
|
With
piperidine; pyridine;
for 0.0833333h;
microwave irradiation;
|
96% |
|
With
piperidine;
In
pyridine;
for 6h;
Heating;
|
95% |
|
piperonal; malonic acid;
With
pyridine;
at 20 ℃;
for 0.166667h;
With
N-methylcyclohexylamine;
for 2h;
Reflux;
|
95% |
|
With
piperidine; pyridine;
for 4h;
Reflux;
|
94% |
|
With
hexamethylenetetramine;
In
water;
for 0.0833333h;
microwave irradiation;
|
93% |
|
With
piperidine; pyridine;
for 30h;
Heating;
|
93% |
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene; 3-amino propanoic acid;
In
ethanol;
at 20 ℃;
stereoselective reaction;
|
92% |
|
With
piperidine; pyridine;
at 22 ℃;
for 3h;
ultrasound;
|
91% |
|
With
piperidine;
In
pyridine;
for 1h;
Heating;
|
86% |
|
piperonal; malonic acid;
With
piperidine; pyridine;
for 3h;
Heating;
With
hydrogenchloride;
at 0 ℃;
|
85% |
|
With
piperidine; pyridine;
for 4h;
Reflux;
|
85% |
|
With
piperidine;
In
pyridine;
for 4h;
Reflux;
|
85% |
|
With
piperidine; pyridine;
for 4h;
Reflux;
|
84% |
|
piperonal; malonic acid;
With
pyridine;
at 20 ℃;
for 0.166667h;
With
piperidine;
for 4h;
Reflux;
|
84% |
|
piperonal; malonic acid;
With
pyridine;
at 20 ℃;
for 0.166667h;
With
piperidine;
for 4h;
Reflux;
|
84% |
|
With
piperidine;
In
acetic acid;
for 0.0333333h;
microwave irradiation;
|
81% |
|
With
piperidine; pyridine;
In
toluene;
at 110 ℃;
for 12h;
Dean-Stark;
|
80.7% |
|
With
piperidine; pyridine;
at 115 ℃;
for 2h;
|
77.1% |
|
With
piperidine; pyridine;
at 115 ℃;
for 2h;
|
77.1% |
|
piperonal; malonic acid;
With
piperidine; pyridine;
at 20 - 100 ℃;
for 3.75h;
With
hydrogenchloride;
In
ethanol; water;
pH=4 - 5;
|
75% |
|
piperonal; malonic acid;
With
piperidine; pyridine;
Reflux;
With
hydrogenchloride;
In
water;
|
75% |
|
With
piperidine; pyridine;
at 90 ℃;
for 1h;
|
73% |
|
With
piperidine; pyridine;
for 8h;
Reflux;
|
69.5% |
|
With
piperidine;
In
pyridine;
at 85 - 105 ℃;
for 4h;
|
68% |
|
With
piperidine; pyridine;
|
|
|
With
ammonia;
|
|
|
With
ammonia;
|
|
|
With
acetic acid;
|
|
|
With
quinoline;
|
|
|
With
piperidine; pyridine;
|
|
|
With
aniline;
In
pyridine;
at 55 ℃;
for 16h;
|
21 mg |
|
|
|
|
With
piperidine; pyridine;
for 8h;
Heating;
|
|
|
With
piperidine; pyridine;
for 4h;
Reflux;
|
|
|
With
pyridine;
at 90 ℃;
|
|
|
With
piperidine; pyridine;
for 2h;
Reflux;
|
|
|
With
piperidine; pyridine;
at 80 ℃;
for 24h;
|
|
|
With
ammonia;
|
|
|
With
piperidine; pyridine;
at 80 - 90 ℃;
for 24h;
|
|
|
With
piperidine; pyridine;
at 80 ℃;
for 24h;
|
|
|
With
morpholine; pyridine;
Reflux;
|
-
-
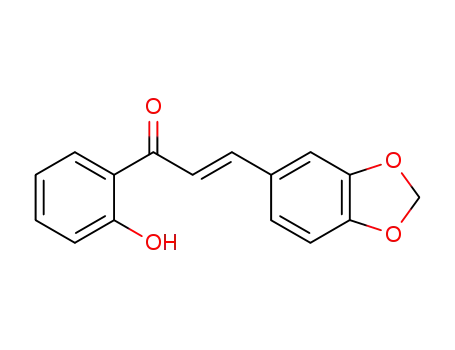
16669-99-1
(E)-2'-hydroxy-3,4-methylenedioxychalcone

-

-
2373-80-0,38489-76-8
3,4-methylenedioxy-trans-cinnamic acid
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; potassium carbonate;
In
acetonitrile;
at 20 ℃;
for 5h;
|
89% |
2373-80-0 Upstream products
-
120-57-0

piperonal
-
141-82-2

malonic acid
-
108-24-7
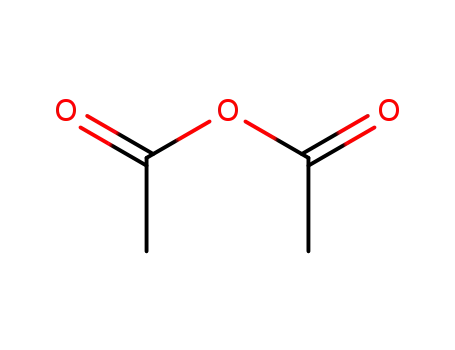
acetic anhydride
-
867-13-0
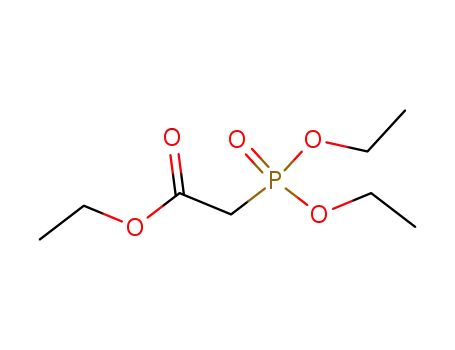
diethoxyphosphoryl-acetic acid ethyl ester
2373-80-0 Downstream products
-
40918-96-5
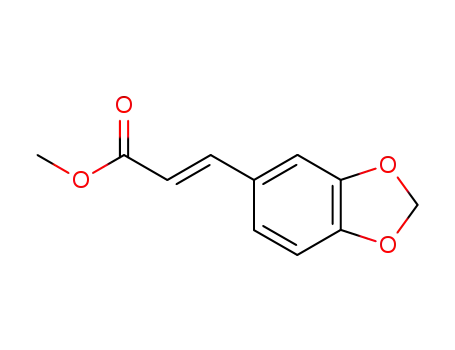
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate
-
14731-78-3
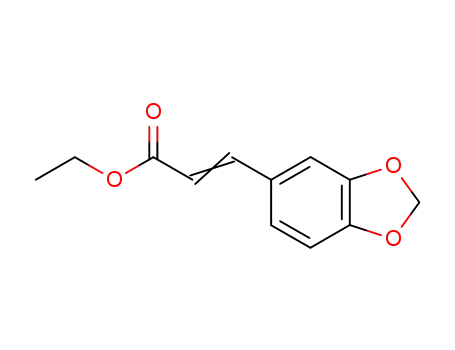
ethyl 3,4-methylenedioxycinnamate
-
495-86-3
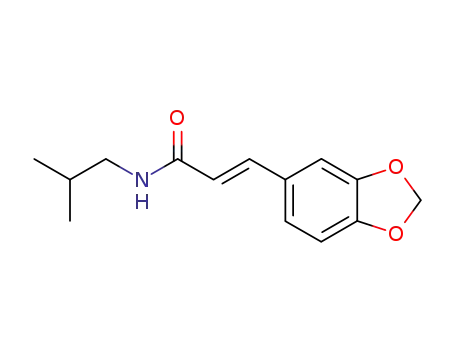
trans-fagaramide
-
62681-68-9
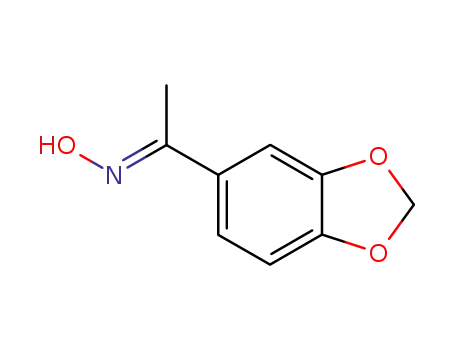
(E)-1-(benzo[d][1,3]dioxol-5-yl)ethan-1-one oxime
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
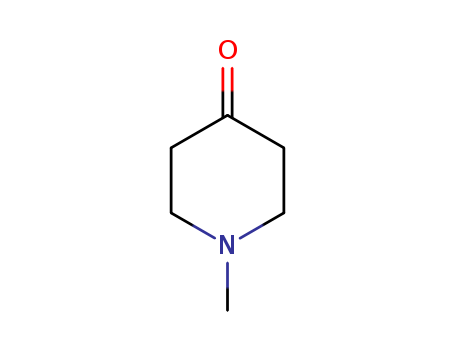
1-Methyl-4-piperidone
CAS:1445-73-4
-
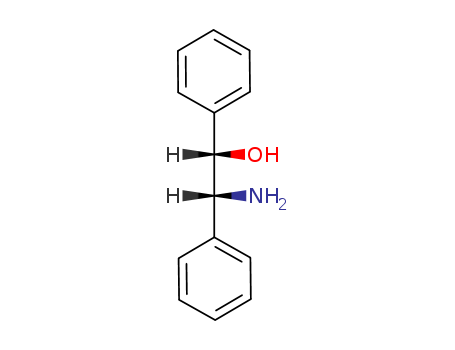
(1S,2R)-2-Amino-1,2-diphenylethanol
CAS:23364-44-5