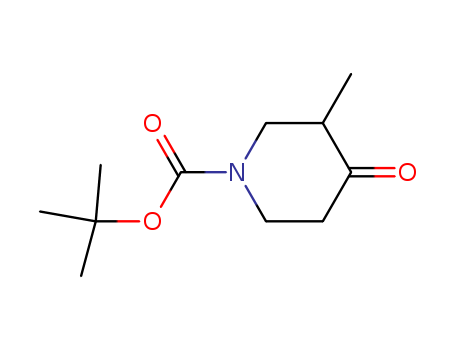
181269-69-2
- Product Name:tert-Butyl 3-methyl-4-oxopiperidine-1-carboxylate
- Molecular Formula:C11H19NO3
- Purity:99%
- Molecular Weight:213.277
Product Details
Chemical plants supply high-quality tert-Butyl 3-methyl-4-oxopiperidine-1-carboxylate 181269-69-2 in bulk
- Molecular Formula:C11H19NO3
- Molecular Weight:213.277
- Vapor Pressure:0.001mmHg at 25°C
- Refractive Index:1.471
- Boiling Point:298.758 °C at 760 mmHg
- PKA:-1.54±0.40(Predicted)
- Flash Point:134.485 °C
- PSA:46.61000
- Density:1.06 g/cm3
- LogP:1.77030
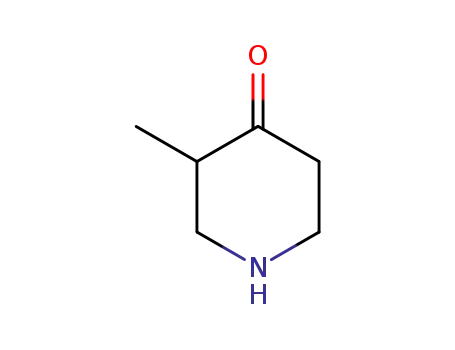
1-BOC-3-METHYL-PIPERIDIN-4-ONE(Cas 181269-69-2) Usage
|
General Description |
1-BOC-3-METHYL-PIPERIDIN-4-ONE is a chemical compound with the molecular formula C12H21NO3. It is a derivative of piperidin-4-one, which is a heterocyclic organic compound with a six-membered ring containing one nitrogen atom. The "1-BOC" in the name indicates the presence of a tert-butoxycarbonyl (BOC) protecting group at the 1-position of the piperidin-4-one ring. This protecting group is commonly used in organic synthesis to prevent unwanted reactions at specific sites in a molecule. 1-BOC-3-METHYL-PIPERIDIN-4-ONE is used as a building block in the synthesis of various pharmaceuticals, agrochemicals, and other organic compounds. It is also a useful intermediate in the preparation of complex organic molecules. |
InChI:InChI=1/C11H19NO3/c1-8-7-12(6-5-9(8)13)10(14)15-11(2,3)4/h8H,5-7H2,1-4H3
181269-69-2 Relevant articles
Selective α-Methylation of Ketones
Frolov, Andriy I.,Ostapchuk, Eugeniy N.,Pashenko, Alexander E.,Chuchvera, Yaroslav O.,Rusanov, Eduard B.,Volochnyuk, Dmitriy M.,Ryabukhin, Sergey V.
, p. 7333 - 7346 (2021/06/28)
The convenient and scalable preparative ...
QUINAZOLINE COMPOUND FOR EGFR INHIBITION
-
Paragraph 0249-0250, (2019/11/21)
Disclosed is a novel quinazoline compoun...
OXADIAZEPINONE DERIVATIVES AND THEIR USE IN THE TREATMENT OF HEPATITIS B INFECTIONS
-
Page/Page column 126, (2018/02/28)
Provided herein are compounds of formula...
NRF2 ACTIVATOR
-
Page/Page column 98-99, (2018/07/29)
Provided are compounds of Formula I, or ...
181269-69-2 Process route
-
-
5773-58-0
3-methyl-4-piperidone

-
-
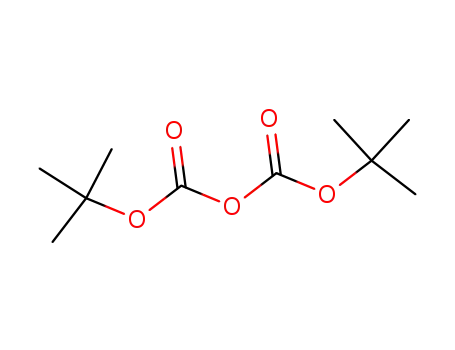
24424-99-5
di-tert-butyl dicarbonate

-
-
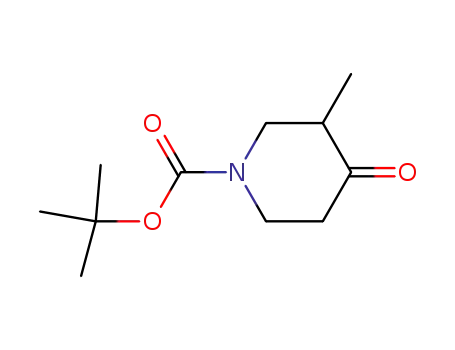
181269-69-2
tert-butyl 3-methyl-4-oxopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
N,N-dimethylethylenediamine;
In
dichloromethane;
at 20 ℃;
for 2.5h;
|
|
|
With
dmap; triethylamine;
In
tetrahydrofuran; water;
at 20 ℃;
for 18h;
|
-
-
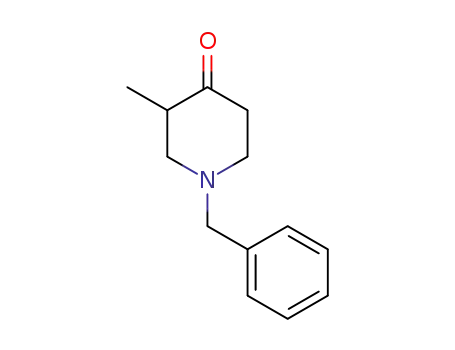
34737-89-8
1-Benzyl-3-methyl-4-piperidon

-

-
24424-99-5
di-tert-butyl dicarbonate

-

-
181269-69-2
tert-butyl 3-methyl-4-oxopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium dihydroxide;
In
ethyl acetate;
for 24h;
under 2068.65 Torr;
|
100% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
at 20 ℃;
for 4h;
under 2585.81 Torr;
|
97% |
|
With
hydrogen;
palladium(II) hydroxide;
In
methanol;
at 20 ℃;
under 2844.39 Torr;
|
95% |
|
With
palladium on activated charcoal; hydrogen;
In
methanol;
at 20 ℃;
for 16h;
under 2585.81 Torr;
|
95.33% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
for 4h;
under 2585.81 Torr;
Inert atmosphere;
|
92% |
|
With
palladium on activated charcoal; hydrogen;
In
methanol;
for 12h;
under 37503.8 Torr;
|
88% |
|
With
hydrogen;
palladium dihydroxide;
In
methanol;
under 2844.39 Torr;
|
81% |
|
With
hydrogen;
palladium hydroxide on carbon;
In
methanol;
under 2844.39 Torr;
|
81% |
|
With
hydrogen; palladium(II) hydroxide;
In
ethanol;
at 20 ℃;
for 6h;
under 5171.62 Torr;
Autoclave;
|
78% |
|
With
hydrogen;
palladium dihydroxide;
In
methanol;
|
|
|
With
hydrogen;
palladium dihydroxide;
In
methanol;
|
|
|
With
hydrogen; palladium(II) hydroxide;
In
ethyl acetate;
at 20 ℃;
under 2250.23 Torr;
|
2.2 g |
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
ethyl acetate;
at 20 ℃;
under 2250.23 Torr;
|
2.2 g |
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
ethyl acetate;
for 12h;
under 2585.81 Torr;
|
|
|
With
10 wt% Pd(OH)2 on carbon;
In
methanol;
|
|
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
at 40 ℃;
for 20h;
under 2327.23 Torr;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
at 30 ℃;
for 5h;
under 2585.81 Torr;
|
181269-69-2 Upstream products
-
5773-58-0

3-methyl-4-piperidone
-
24424-99-5

di-tert-butyl dicarbonate
-
34737-89-8

1-Benzyl-3-methyl-4-piperidon
-
79099-07-3
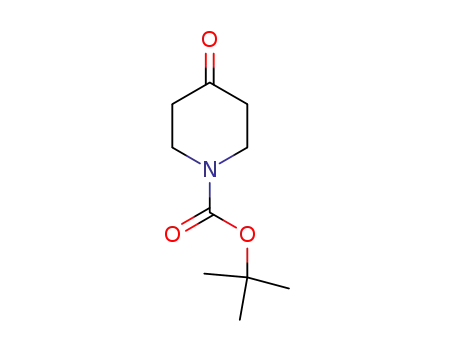
N-tert-butyloxycarbonylpiperidin-4-one
181269-69-2 Downstream products
-
181269-70-5
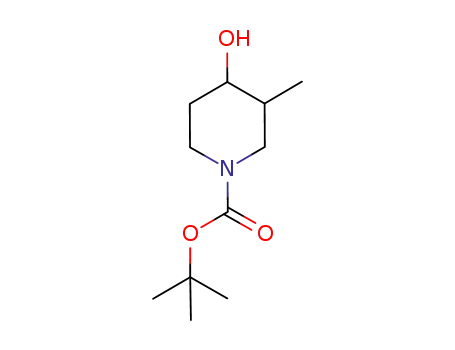
trans-tert-butyl-4-hydroxy-3-methylpiperidine-1-carboxylate
-
723308-57-4
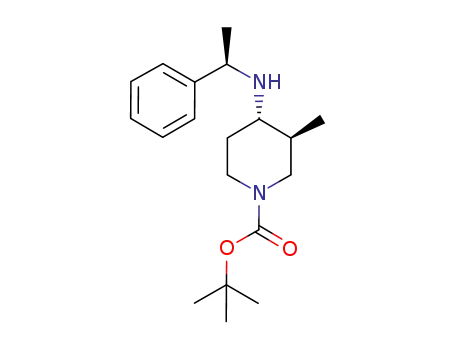
(3S,4S)-3-methyl-4-(1-(R)-phenyl-ethylamino)-piperidine-1-carboxylic acid tert-butyl ester
-
723308-56-3
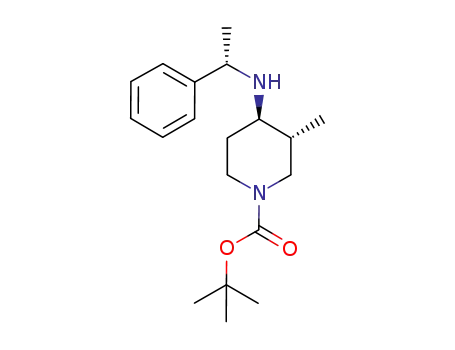
(3R,4R)-3-methyl-4-(1-(S)-phenyl-ethylamino)-piperidine-1-carboxylic acid tert-butyl ester
-
1428341-13-2
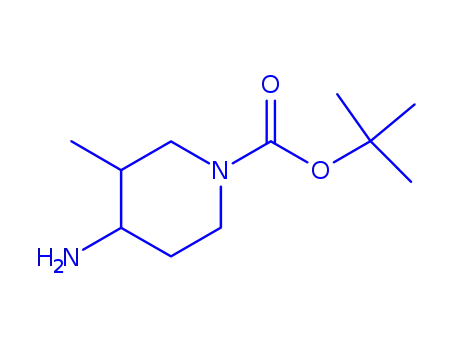
tert-butyl cis-4-amino-3-methyl-1-piperidinecarboxylate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
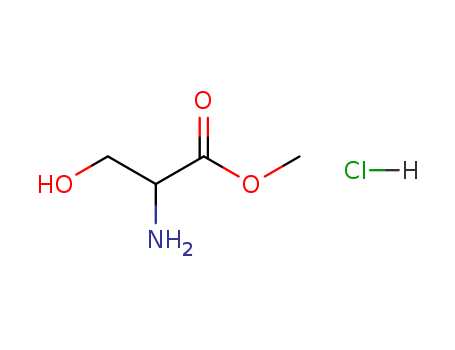
Methyl-DL-serine hydrochloride
CAS:5619-04-5
-
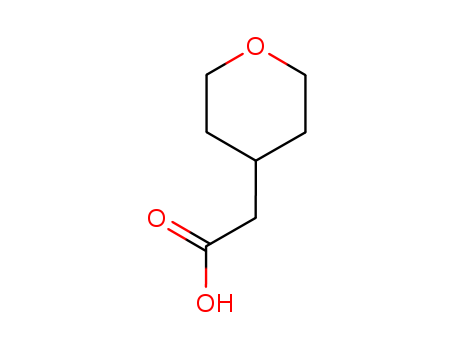
2-(tetrahydro-2H-pyran-4-yl)acetic acid
CAS:85064-61-5