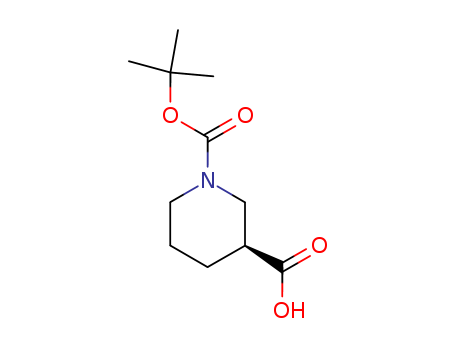
88495-54-9
- Product Name:L-1-Boc-Nipecotic acid
- Molecular Formula:C11H19NO4
- Purity:99%
- Molecular Weight:229.276
Product Details
pd_meltingpoint:159-162 °C(lit.)
Appearance:White solid
Chemical plants supply high-quality L-1-Boc-Nipecotic acid 88495-54-9 in bulk
- Molecular Formula:C11H19NO4
- Molecular Weight:229.276
- Appearance/Colour:White solid
- Vapor Pressure:6.15E-06mmHg at 25°C
- Melting Point:159-162 °C(lit.)
- Refractive Index:1.496
- Boiling Point:353.2 °C at 760 mmHg
- PKA:4.49±0.20(Predicted)
- Flash Point:167.4 °C
- PSA:66.84000
- Density:1.164 g/cm3
- LogP:1.65600
L-1-Boc-Nipecotic acid(Cas 88495-54-9) Usage
InChI:InChI=1/C11H19NO4/c1-11(2,3)16-10(15)12-6-4-5-8(7-12)9(13)14/h8H,4-7H2,1-3H3,(H,13,14)/t8-/m0/s1
88495-54-9 Relevant articles
Design, Synthesis and Enhanced BBB Penetration Studies of L-serine-Tethered Nipecotic Acid-Prodrug
Dhanawat, Meenakshi,Gupta, Sumeet,Mehta, Dinesh Kumar,Das, Rina
, p. 94 - 103 (2020/12/21)
Nipecotic acid is considered to be one o...
NAPHTHO[2,1 -D]THIAZOLE DERIVATIVES, COMPOSITIONS THEREOF AND METHODS OF TREATING DISORDERS
-
Paragraph 0115, (2021/05/29)
The present application relates to the c...
Design and evaluation of novel piperidine HIV-1 protease inhibitors with potency against DRV-resistant variants
Zhu, Mei,Zhou, Huiyu,Ma, Ling,Dong, Biao,Zhou, Jinming,Zhang, Guoning,Wang, Minghua,Wang, Juxian,Cen, Shan,Wang, Yucheng
, (2021/06/02)
A novel class of HIV-1 protease inhibito...
PYRAZOLOPYRIDAZINE DERIVATIVES, PREPARATION METHOD THEREOF AND COMPOSITION FOR PREVENTING OR TREATING CANCER COMPRISING THE SAME
-
Paragraph 0245-0246; 0251-0253, (2020/11/14)
The present invention is a pyrazolopyrid...
88495-54-9 Process route
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
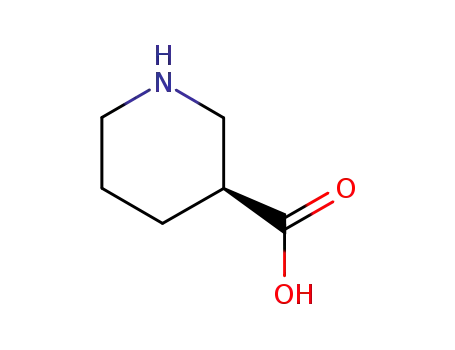
498-95-3,25137-00-2,59045-82-8,60252-41-7,262853-93-0
(S)-nipecotic acid

-
-
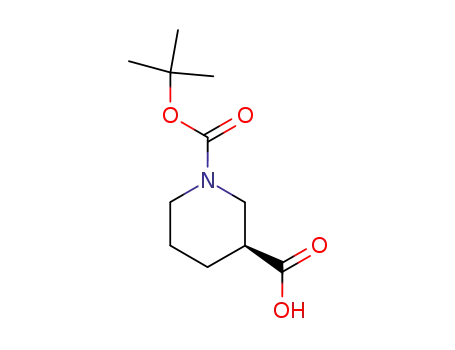
88495-54-9
(3S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
Inert atmosphere;
|
95% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 25 ℃;
Inert atmosphere;
|
94.8% |
|
With
triethylamine;
In
methanol;
at 0 - 20 ℃;
for 23h;
Inert atmosphere;
|
92% |
|
di-tert-butyl dicarbonate; (S)-nipecotic acid;
With
triethylamine;
In
methanol;
at 0 - 20 ℃;
for 23h;
Inert atmosphere;
With
potassium hydrogensulfate;
In
water;
at 0 ℃;
pH=2;
|
92% |
|
With
sodium carbonate;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
for 14h;
Inert atmosphere;
|
87% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 25 ℃;
Inert atmosphere;
|
-
-
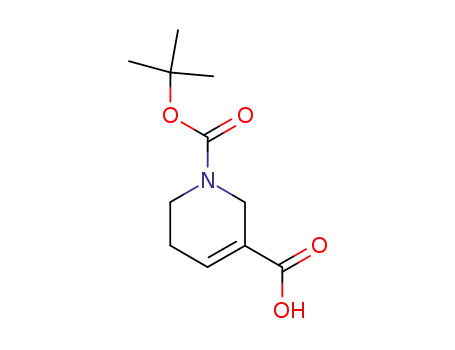
86447-11-2
1-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-3-carboxylic acid

-

-
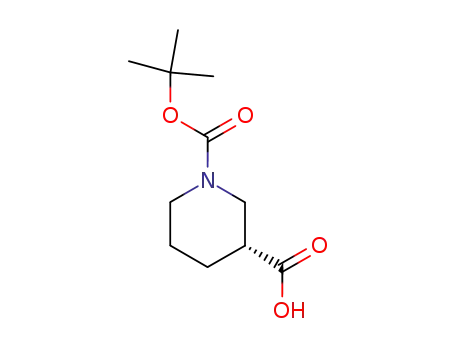
88495-54-9
(3S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid

-
-
163438-09-3
(R)-1-Boc-nipecotic acid
| Conditions | Yield |
|---|---|
|
With
C51H54IrNOP(1+)*C32H12BF24(1-); hydrogen; caesium carbonate;
In
methanol;
at 60 ℃;
for 12h;
under 4560.31 Torr;
enantioselective reaction;
Autoclave;
|
82 % ee |
88495-54-9 Upstream products
-
148672-74-6
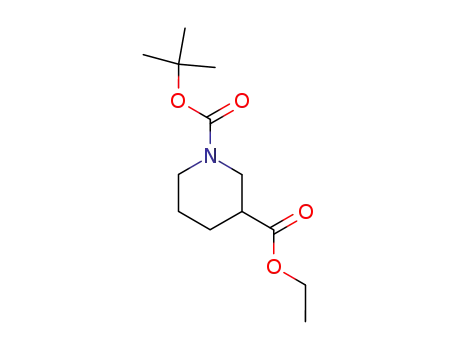
ethyl N-(t-butyloxycarbonyl)nipecotate
-
58632-95-4
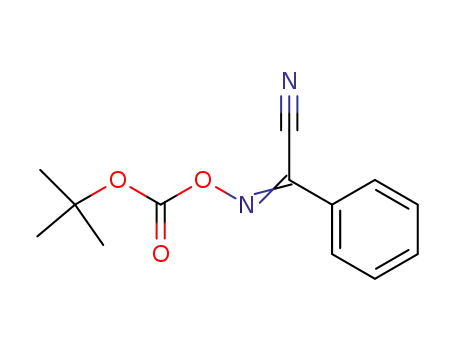
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile
-
498-95-3
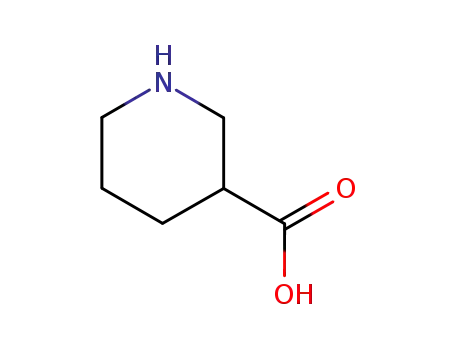
nipecotic acid
-
24424-99-5

di-tert-butyl dicarbonate
88495-54-9 Downstream products
-
84358-18-9
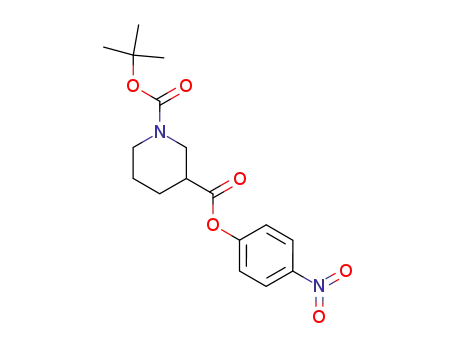
p-nitrophenyl 1-(tert-butoxycarbonyl)-3-piperidinecarboxylate
-
93801-23-1
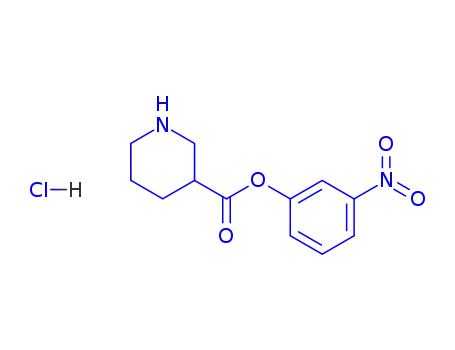
m-nitrophenyl 3-piperidinecarboxylate hydrochloride
-
91419-49-7
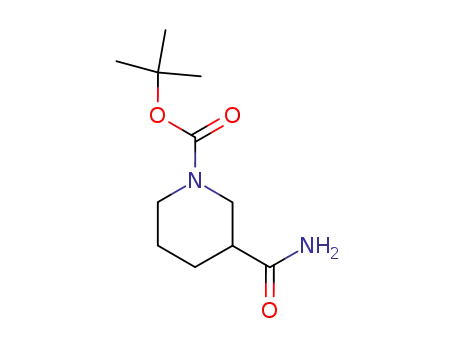
tert-butyl 3-carbamoylpiperidine-1-carboxylate
-
152170-49-5

N-(t-butyloxycarbonyl)piperidine-3-carboxylic acid N-methylanilide
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
TETRAHYDROPYRAN-2-CARBOXYLIC ACID
CAS:51673-83-7
-
4-Dimethylaminopyridine
CAS:1122-58-3