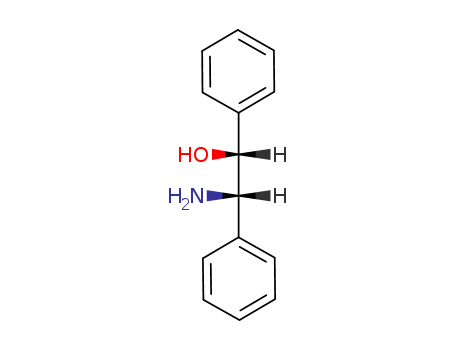
23190-16-1
- Product Name:(1R,2S)-2-Amino-1,2-diphenylethanol
- Molecular Formula:C14H15NO
- Purity:99%
- Molecular Weight:213.279
Product Details
pd_meltingpoint:142-144 °C(lit.)
Appearance:white to light yellow crystal powder
Top quality factory supply 23190-16-1 (1R,2S)-2-Amino-1,2-diphenylethanol at low price
- Molecular Formula:C14H15NO
- Molecular Weight:213.279
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:2.88E-06mmHg at 25°C
- Melting Point:142-144 °C(lit.)
- Refractive Index:-7 ° (C=0.6, EtOH)
- Boiling Point:261 °C at 760 mmHg
- PKA:11.70±0.45(Predicted)
- Flash Point:125.3 °C
- PSA:46.25000
- Density:1.148 g/cm3
- LogP:3.12030
(1R,2S)-2-Amino-1,2-diphenylethanol(Cas 23190-16-1) Usage
InChI:InChI=1/C14H15NO/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14,16H,15H2/t13-,14+/m0/s1
23190-16-1 Relevant articles
Double Asymmetric Hydrogenation of α-Iminoketones: Facile Synthesis of Enantiopure Vicinal Amino Alcohols
Lin, Xin,Shao, Pan-Lin,Song, Jingyuan,Wang, Jiang,Wen, Jialin,Zhang, Xumu
, p. 12729 - 12735 (2021/10/29)
This study presents an Rh/DuanPhos-catal...
NOVEL METHOD FOR PREPARING (2R)-2-(2-METHOXYPHENYL)-2-(OXANE-4-YLOXY)ETHANE-1-OL COMPOUND, AND INTERMEDIATE USED THEREIN
-
Paragraph 0097-0101, (2020/09/22)
A novel method for preparing a compound ...
Site-Specific C(sp3)–H Aminations of Imidates and Amidines Enabled by Covalently Tethered Distonic Radical Anions
Fang, Yuanding,Fu, Kang,Shi, Lei,Zhao, Rong,Zhou, Jia
supporting information, p. 20682 - 20690 (2020/09/07)
The utilization of N-centered radicals t...
Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides
Marcyk, Paul T.,Jefferies, Latisha R.,AbuSalim, Deyaa I.,Pink, Maren,Baik, Mu-Hyun,Cook, Silas P.
supporting information, p. 1727 - 1731 (2019/01/21)
The direct, catalytic substitution of un...
23190-16-1 Process route
-
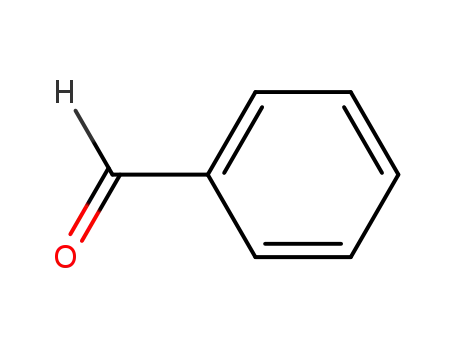
-
100-52-7
benzaldehyde

-
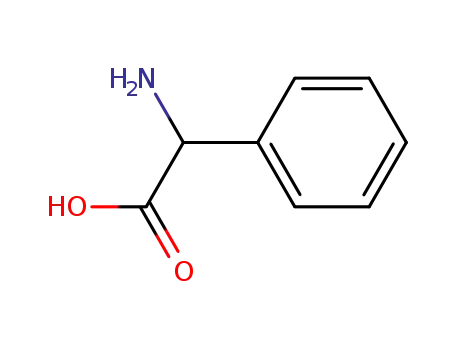
-
2835-06-5
phenylglycin

-
-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
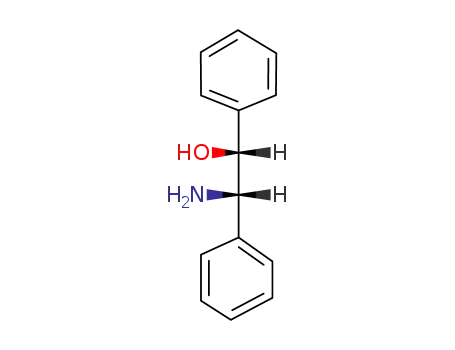
erythro-2-amino-1,2-diphenylethanol

-
-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
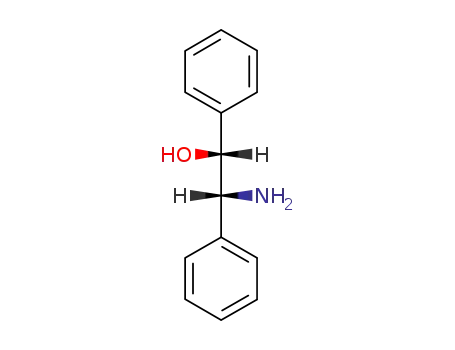
threo-1,2-diphenyl-2-aminoethan-1-ol

-
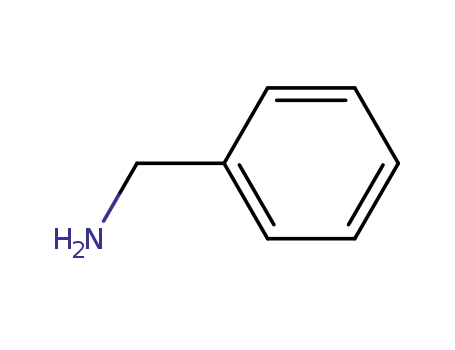
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
|
-

-
100-52-7
benzaldehyde

-

-
2835-06-5
phenylglycin

-

-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
erythro-2-amino-1,2-diphenylethanol

-

-
530-36-9,3764-63-4,13286-63-0,23190-16-1,23190-17-2,23364-44-5,23412-95-5,39664-87-4,88082-66-0
threo-1,2-diphenyl-2-aminoethan-1-ol

-
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
|
23190-16-1 Upstream products
-
64-17-5
ethanol
-
87-69-4
L-Tartaric acid
-
530-36-9

threo-1,2-diphenyl-2-aminoethan-1-ol
-
572-45-2
benzil dioxime
23190-16-1 Downstream products
-
92552-75-5
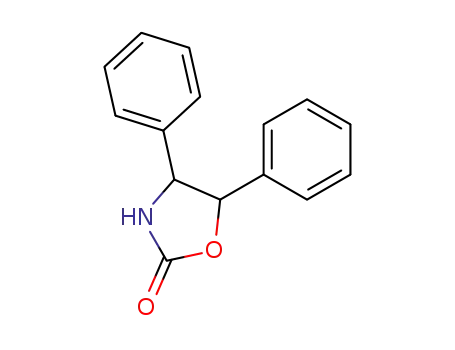
4,5-diphenyl-2-oxazolidinone
-
6275-45-2
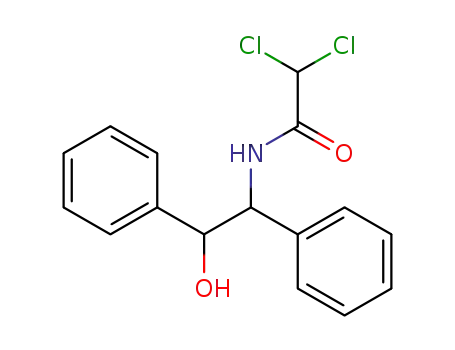
dichloro-acetic acid-(α'-hydroxy-bibenzyl-α-ylamide)
-
530-36-9
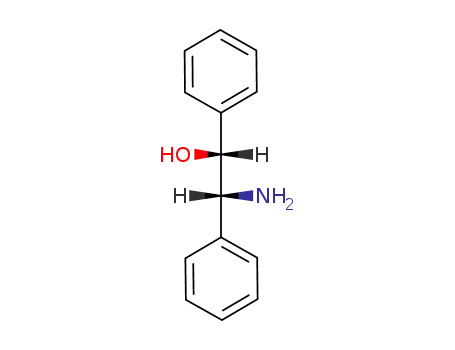
(1R,2R)-2-amino-1,2-diphenylethane-1-ol
-
492-70-6
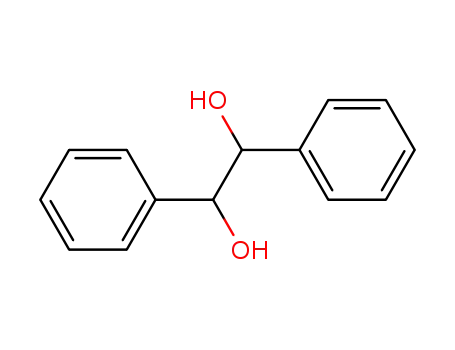
1,2-diphenyl-1,2-ethanediol
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
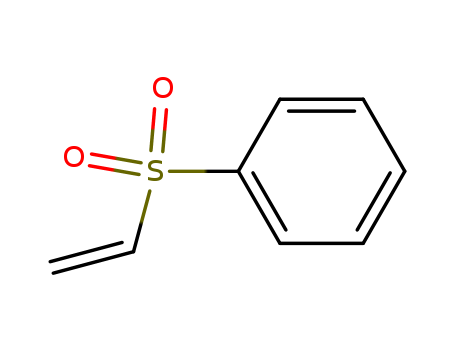
Phenyl vinyl sulfone
CAS:5535-48-8