495-76-1
- Product Name:Piperonyl alcohol
- Molecular Formula:C8H8O3
- Purity:99%
- Molecular Weight:152.15
Product Details
pd_meltingpoint:50-54 °C(lit.)
Appearance:White crystals or crystalline powder
Factory Sells Best Quality Piperonyl alcohol 495-76-1 with ISO standards
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:White crystals or crystalline powder
- Vapor Pressure:0.00161mmHg at 25°C
- Melting Point:50-54 °C(lit.)
- Refractive Index:1.594
- Boiling Point:282.2 °C at 760 mmHg
- PKA:14.36±0.10(Predicted)
- Flash Point:124.5 °C
- PSA:38.69000
- Density:1.329 g/cm3
- LogP:0.90760
Piperonyl alcohol(Cas 495-76-1) Usage
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 71, p. 3657, 1949 DOI: 10.1021/ja01179a022 |
InChI:InChI=1/C8H8O3/c9-4-6-1-2-7-8(3-6)11-5-10-7/h1-3,9H,4-5H2
495-76-1 Relevant articles
A novel method to convert ketones and aldehydes to the corresponding alcohols
Wei-Dong, Yang,Chi, Yang,An-Xing, Wu
, p. 2827 - 2830 (1998)
A novel method of converting aldehydes a...
Degradation of the neolignan, burchellin in the hemolymph of the bloodsucking insect Rhodnius prolixus
Cabral, Marise M.O.,Garcia, Eloi S.,Gottlieb, Otto R.,Kelecom, Alphonse
, p. 59 - 63 (2008)
The neolignan, burchellin, a natural com...
Iron-catalyzed chemoselective hydride transfer reactions
Coufourier, Sébastien,Ndiaye, Daouda,Gaillard, Quentin Gaignard,Bettoni, Léo,Joly, Nicolas,Mbaye, Mbaye Diagne,Poater, Albert,Gaillard, Sylvain,Renaud, Jean-Luc
supporting information, (2021/06/07)
A Diaminocyclopentadienone iron tricarbo...
Efficient and chemoselective hydrogenation of aldehydes catalyzed by well-defined PN3-pincer manganese(ii) catalyst precursors: An application in furfural conversion
Gholap, Sandeep Suryabhan,Dakhil, Abdullah Al,Chakraborty, Priyanka,Li, Huaifeng,Dutta, Indranil,Das, Pradip K.,Huang, Kuo-Wei
supporting information, p. 11815 - 11818 (2021/11/30)
Well-defined and air-stable PN3-pincer m...
Light-driven MPV-type reduction of aryl ketones/aldehydes to alcohols with isopropanol under mild conditions
Cao, Dawei,Xia, Shumei,Pan, Pan,Zeng, Huiying,Li, Chao-Jun,Peng, Yong
supporting information, p. 7539 - 7543 (2021/10/12)
Alcohols are versatile structural motifs...
Method for synthesizing piperonyl alcohol through catalytic hydrogenation
-
Paragraph 0024-0035, (2021/02/10)
The invention relates to the technical f...
495-76-1 Process route
-
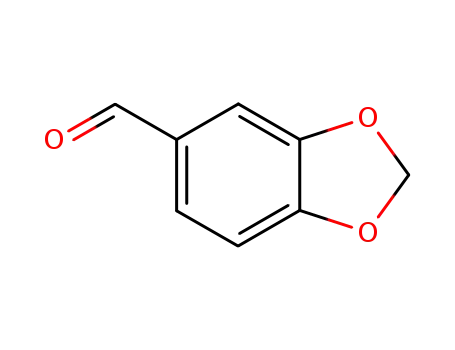
-
120-57-0,30024-74-9
piperonal

-
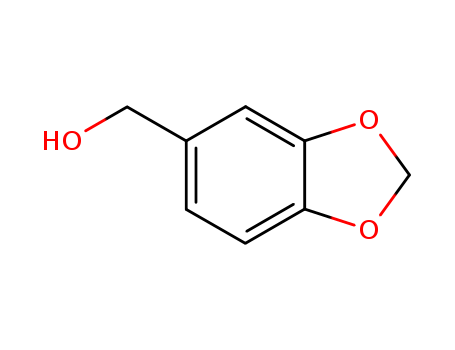
-
495-76-1
piperonol
| Conditions | Yield |
|---|---|
|
With
diisopropoxytitanium(III) tetrahydroborate;
In
dichloromethane;
at -20 ℃;
for 0.133333h;
|
100% |
|
With
Zn(BH4)2(Ph3P)2;
In
tetrahydrofuran;
at 20 ℃;
|
100% |
|
With
sodium tetrahydroborate;
In
methanol;
at 20 ℃;
for 1h;
|
100% |
|
With
hydrogen;
In
water;
at 40 ℃;
for 0.00611111h;
under 22502.3 Torr;
chemoselective reaction;
Flow reactor;
Green chemistry;
|
99% |
|
With
trimethylamine-N-oxide; (1,4-dimethyl-5,7-diphenyl-1,2,3,4-tetrahydro-6H-cyclopenta[b]pyrazin-6-one) irontricarbonyl complex3; potassium formate;
In
ethanol;
at 45 - 60 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
99% |
|
antimony(III) chloride; aluminium;
In
water; N,N-dimethyl-formamide;
for 1h;
Ambient temperature;
|
98% |
|
With
water; antimony(III) chloride; aluminium;
In
N,N-dimethyl-formamide;
for 1h;
Ambient temperature;
|
98% |
|
With
water; nickel dichloride; zinc;
In
N,N-dimethyl-formamide;
for 1h;
Ambient temperature;
|
98% |
|
With
cobalt(II) chloride; zinc;
In
water; N,N-dimethyl-formamide;
for 1.5h;
Ambient temperature;
|
98% |
|
With
bis(triphenylphosphine)copper(I) nitrate; hydrogen; 1,4-di(diphenylphosphino)-butane; sodium hydroxide;
In
ethanol;
at 50 ℃;
for 16h;
under 37503.8 Torr;
chemoselective reaction;
Autoclave;
Inert atmosphere;
|
98% |
|
With
sodium tetrahydroborate;
In
methanol;
at 0 ℃;
for 0.25h;
Inert atmosphere;
|
98% |
|
With
hydrogen; sodium hydroxide;
bis(triphenylphosphane)copper(I) nitrate; 1,4-di(diphenylphosphino)-butane;
In
ethanol;
at 50 ℃;
for 16h;
under 37503.8 Torr;
Autoclave;
Inert atmosphere;
|
98% |
|
With
hydrogen; sodium hydroxide;
bis(triphenylphosphane)copper(I) nitrate; 1,4-di(diphenylphosphino)-butane;
In
ethanol;
at 50 ℃;
for 16h;
under 37503.8 Torr;
Autoclave;
Inert atmosphere;
|
98% |
|
With
methanol; sodium tetrahydroborate;
at 0 - 20 ℃;
for 0.5h;
|
98% |
|
With
sodium tetrahydroborate; sodium hydroxide;
In
methanol; water;
at 20 ℃;
for 0.833333h;
|
97% |
|
With
hydrogen;
In
methanol;
at 20 ℃;
for 4h;
chemoselective reaction;
|
97% |
|
With
(1,4-diazabicyclo{2.2.2}-octane)zinc(II) tetrahydoborate;
In
tetrahydrofuran;
for 6.5h;
Ambient temperature;
|
96% |
|
With
cyclopentylmagnesium bromide;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
|
95% |
|
With
trimethylamine-N-oxide; sodium formate; C34H44FeN4O4(2+)*2I(1-);
In
water;
at 80 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
95% |
|
With
diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; tris[3,5-bis(trifluoromethyl)phenyl]-borane;
In
1,4-dioxane;
at 25 ℃;
for 12h;
Glovebox;
|
94% |
|
With
ethanol; aluminium; sodium iodide;
for 6h;
Electrolysis;
refluxe;
|
93% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 40 ℃;
for 3h;
|
92.8% |
|
With
sodium tetrahydroborate; ethanol;
at 40 ℃;
for 3h;
|
92.8% |
|
With
methyltriphenylphosphonium tetrahydroborate;
In
dichloromethane;
|
91% |
|
With
Decaborane;
In
tetrahydrofuran; water;
at 20 ℃;
for 0.5h;
|
91% |
|
piperonal;
With
polymethylhydrosiloxane; iron(II) acetate; tricyclohexylphosphine;
In
tetrahydrofuran;
at 65 ℃;
for 16h;
With
sodium hydrogencarbonate;
In
tetrahydrofuran; methanol;
at 0 - 20 ℃;
Further stages.;
|
91% |
|
With
sulfurated borohydride exchange resin;
In
methanol;
at 25 ℃;
for 0.166667h;
|
90% |
|
With
triethylsilane; water; palladium diacetate;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 1h;
|
90% |
|
With
sodium tetrahydroborate;
In
methanol;
at 0 - 25 ℃;
for 4h;
|
90% |
|
With
platinum on activated charcoal; hydrogen;
In
ethanol;
at 20 ℃;
for 3h;
|
89% |
|
With
potassium fluoride on basic alumina; formaldehyd;
for 0.116667h;
microwave irradiation;
|
88% |
|
With
sodium tetrahydroborate; nickel dichloride;
In
tetrahydrofuran;
at 20 ℃;
for 0.0833333h;
|
86% |
|
With
magnesium; tin(ll) chloride;
In
tetrahydrofuran;
for 0.25h;
|
85% |
|
With
potassium tert-butylate; hydrogen; C18H26Br2MnN3P;
In
methanol;
under 20252 Torr;
chemoselective reaction;
Inert atmosphere;
Autoclave;
|
85% |
|
With
hexarhodium hexadecacarbonyl; carbon monoxide; aminated polymer; water;
In
benzene;
at 80 ℃;
for 24h;
under 11400 Torr;
|
83% |
|
With
indium isopropoxide; isopropyl alcohol;
for 2h;
Inert atmosphere;
|
83% |
|
With
hydrogen;
copper chromite;
In
1,4-dioxane;
at 170 - 175 ℃;
for 2h;
under 136800 - 174800 Torr;
Product distribution;
other temp., pressure; other substrates, reagents;
|
80% |
|
With
hydrogen;
copper chromite;
In
1,4-dioxane;
at 170 - 175 ℃;
for 2h;
under 136800 - 174800 Torr;
|
80% |
|
piperonal;
With
1-Methylpyrrolidine; 2-chloro-5-fluorophenylboronic acid; phenylsilane;
at 20 ℃;
for 16h;
Inert atmosphere;
With
sodium hydroxide;
In
water;
at 20 ℃;
for 2h;
chemoselective reaction;
|
74% |
|
With
lithium tert-butoxide;
In
isopropyl alcohol;
at 20 ℃;
for 36h;
UV-irradiation;
Inert atmosphere;
|
73% |
|
With
phenylselenomagnesium bromide;
In
tetrahydrofuran;
for 13h;
Ambient temperature;
|
56.1% |
|
With
hydrazine hydrate;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
Sealed tube;
|
49% |
|
With
triethylsilane; zinc(II) iodide;
In
1,2-dichloro-ethane;
at 20 ℃;
for 6h;
|
30% |
|
With
lithium aluminium tetrahydride; diethyl ether;
|
|
|
With
acetic acid ester; hydrogen;
weitere Reagens Platinschwarz;
|
|
|
With
ethanol; hydrogen;
weitere Reagens Platinschwarz;
|
|
|
With
platinum(IV) oxide; ethanol; iron(II) chloride;
under 2280 Torr;
Hydrogenation;
|
|
|
With
Pd-BaSO4; acetic acid;
Hydrogenation;
|
|
|
With
sodium hydrogencarbonate; mercury;
at 10 - 12 ℃;
Electrolysis;
|
|
|
With
methanol;
|
|
|
With
ethanol;
|
|
|
With
methanol; palladium; N,N-diethylnicotinamide;
at 20 ℃;
Hydrogenation;
|
|
|
With
palladium on activated charcoal; isopropyl alcohol;
at 70 - 90 ℃;
under 29420.3 Torr;
|
|
|
With
1,4-dioxane; copper oxide-chromium oxide;
at 160 - 175 ℃;
under 220652 Torr;
Hydrogenation;
|
|
|
With
ethanol; nickel;
at 35 - 60 ℃;
Hydrogenation.unter Druck;
|
|
|
With
potassium hydroxide; benzyl alcohol;
at 205 ℃;
|
|
|
With
aluminum isopropoxide; isopropyl alcohol;
|
|
|
With
potassium hydroxide;
at 20 ℃;
|
|
|
With
nickel; decalin;
at 120 - 130 ℃;
Hydrogenation;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
|
|
|
With
sodium tetrahydroborate;
In
methanol; dichloromethane;
for 1h;
|
|
|
With
methanol; methyltriphenylphosphonium tetrahydroborate;
Yield given. Multistep reaction;
1) CH2Cl2, room temperature, 1 min, 2) 1 h;
|
|
|
With
sodium hydroxide; polymethylhydrosiloxane; tetrabutyl ammonium fluoride;
Yield given. Multistep reaction;
1.) THF, room t., 2.) THF;
|
|
|
With
sodium hydroxide; sodium tetrahydroborate; zinc 2-ethylhexanoate;
Yield given;
Multistep reaction;
1.) THF, reflux, 2.) 40 deg, 1 h;
|
|
|
With
sodium tetrahydroborate; silica gel;
In
hexane;
at 40 ℃;
for 3h;
Yield given;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at 20 ℃;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
|
|
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; water;
at 20 ℃;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
|
|
|
With
sodium tetrahydroborate;
|
|
|
With
potassium tert-butylate; hydrogen;
(N,N'-bis(2-(tert-butylthio-κS)benzylidene)-1,2-ethanediamino-κN,κN')dichlororuthenium(II);
In
isopropyl alcohol;
at 60 ℃;
for 2h;
under 37503.8 Torr;
Product distribution / selectivity;
autoclave;
|
99 %Chromat. |
|
With
sodium tetrahydroborate;
In
dichloromethane;
at 20 ℃;
for 0.5h;
|
|
|
With
(N,N'-bis(2-(tert-butylthio-kS)benzylidene)-1,2-ethanediamino-kN,kN')dichlororuthenium(II); potassium tert-butylate; hydrogen;
In
isopropyl alcohol;
at 60 ℃;
for 2h;
under 37503.8 Torr;
Inert atmosphere;
Autoclave;
|
99 %Chromat. |
|
With
sodium tetrahydroborate;
In
methanol;
at 0 - 20 ℃;
for 0.5h;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at -10 ℃;
|
188.8 mg |
|
With
sodium tetrahydroborate;
In
methanol;
Sealed tube;
|
|
|
With
methanol; sodium tetrahydroborate;
at 20 ℃;
Schlenk technique;
Inert atmosphere;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 25 ℃;
for 0.5h;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
|
|
|
With
[(iPr-PNP)Fe(H)(Br)(CO)]; potassium tert-butylate; hydrogen; triethylamine;
In
ethanol;
at 40 ℃;
for 16h;
under 22502.3 Torr;
Autoclave;
|
94 %Spectr. |
|
With
potassium borohydride;
In
methanol; water;
at 20 ℃;
for 2h;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
for 2h;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at 20 ℃;
Inert atmosphere;
Schlenk technique;
|
|
|
Multi-step reaction with 2 steps
1: C23H33MnN3Si2 / diethyl ether / 16 h / 25 °C / Glovebox; Inert atmosphere
2: silica gel / ethyl acetate; hexane
With
C23H33MnN3Si2; silica gel;
In
diethyl ether; hexane; ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1.1: pyridine / dichloromethane / 0.08 h / 25 °C
1.2: 12 h / 25 °C
2.1: sodium tetrahydroborate / tetrahydrofuran; water / 12 h / 0 - 25 °C
With
pyridine; sodium tetrahydroborate;
In
tetrahydrofuran; dichloromethane; water;
|
|
|
Multi-step reaction with 2 steps
1: sodium metabisulfite / water / 12 h / 20 °C / Cooling with ice
2: sodium tetrahydroborate; ethanol / 0.75 h / 20 °C
With
sodium metabisulfite; sodium tetrahydroborate; ethanol;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / di-isopropyl ether / 6 h / 20 °C
2: sodium tetrahydroborate; ethanol / 0.75 h / 20 °C
With
sodium tetrahydroborate; ethanol; sodium hydroxide;
In
di-isopropyl ether;
|
|
|
Multi-step reaction with 2 steps
1: cis-[(H)(SePh)Fe(PMe3)4] / tetrahydrofuran / 2 h / 50 °C
2: sodium hydroxide; methanol / 50 °C
With
methanol; cis-[(H)(SePh)Fe(PMe3)4]; sodium hydroxide;
In
tetrahydrofuran;
|
|
|
With
zirconium(IV) pyrophosphate;
In
isopropyl alcohol;
at 160 ℃;
for 10h;
|
|
|
Multi-step reaction with 2 steps
1: [CoCl2(4'-(4-pyridyl)-2,2':6',2''-terpyridine)]·2H2O; potassium tert-butylate / tetrahydrofuran / 16 h / 25 °C / Inert atmosphere; Glovebox
2: silica gel / 25 °C / Inert atmosphere; Glovebox
With
[CoCl2(4'-(4-pyridyl)-2,2':6',2''-terpyridine)]·2H2O; potassium tert-butylate; silica gel;
In
tetrahydrofuran;
|
|
|
With
water; nickel;
at 50 - 70 ℃;
under 73550.8 Torr;
Hydrogenation;
|
|
|
Multi-step reaction with 2 steps
1: two-dimensional iron(II) coordination polymer based on a divergent 4'-(4-diphenylamino)phenyl-4,2';6',4''-terpyridine ligand; potassium tert-butylate / neat (no solvent) / 0.5 h / 20 °C / Green chemistry
2: silica gel / ethyl acetate; hexane / 20 °C
With
two-dimensional iron(II) coordination polymer based on a divergent 4'-(4-diphenylamino)phenyl-4,2';6',4''-terpyridine ligand; potassium tert-butylate; silica gel;
In
hexane; ethyl acetate;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at 20 ℃;
for 18h;
|
|
|
Multi-step reaction with 2 steps
1: 0.55C27H43N3Si3V*0.45C27H44N3Si3V / diethyl ether / 2 h / 20 °C / Inert atmosphere; Glovebox; Schlenk technique
2: air / diethyl ether
With
0.55C27H43N3Si3V*0.45C27H44N3Si3V;
In
diethyl ether;
|
|
|
With
hydrogen;
In
ethanol;
at 70 - 80 ℃;
for 4h;
under 375.038 - 15001.5 Torr;
Temperature;
Solvent;
Pressure;
|
100.1 g |
|
With
methanol; sodium tetrahydroborate;
at 0 - 20 ℃;
Inert atmosphere;
|
-
![5-(tetrahydro-2H-pyran-2-yloxy)methylbenzo[d][1,3]dioxole](/upload/2024/12/ae51f423-f34d-448e-889e-885e3b4b4ac4.png)
-
85604-71-3
5-(tetrahydro-2H-pyran-2-yloxy)methylbenzo[d][1,3]dioxole

-

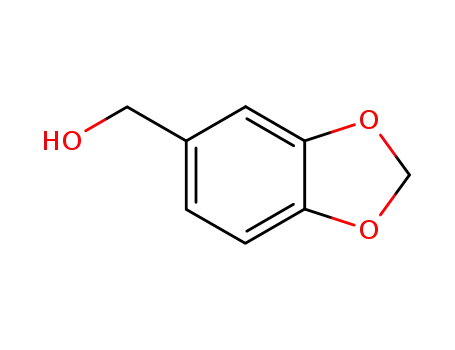
-
495-76-1
piperonol
| Conditions | Yield |
|---|---|
|
With
trichloroisocyanuric acid;
In
methanol;
at 20 ℃;
for 4h;
|
92% |
|
With
cerium(III) chloride;
In
methanol;
at 20 ℃;
for 0.5h;
|
90% |
|
With
carbon tetrabromide;
In
methanol;
at 65 ℃;
for 0.5h;
Heating;
|
89% |
|
With
water; lithium chloride;
In
dimethyl sulfoxide;
at 90 ℃;
for 6h;
|
88% |
|
With
copper dichloride;
In
methanol;
at 20 ℃;
|
87% |
|
With
CuCl2*2H2O;
In
methanol;
at 20 ℃;
for 1.25h;
|
87% |
|
With
tin(ll) chloride;
In
methanol;
|
86% |
|
With
water;
β‐cyclodextrin;
In
methanol;
at 50 ℃;
for 10h;
|
80% |
495-76-1 Upstream products
-
120-57-0

piperonal
-
64-17-5
ethanol
-
91247-76-6
2-(methyl-piperonyl-amino)-ethanol
-
108-24-7
acetic anhydride
495-76-1 Downstream products
-
5442-27-3
5-(1-butoxy-ethoxymethyl)-benzo[1,3]dioxole
-
5469-06-7
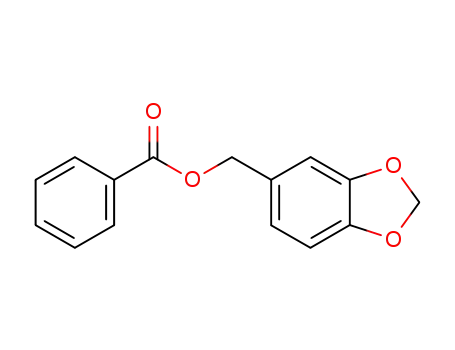
benzo[d][1,3]dioxol-5-ylmethyl benzoate
-
5432-93-9
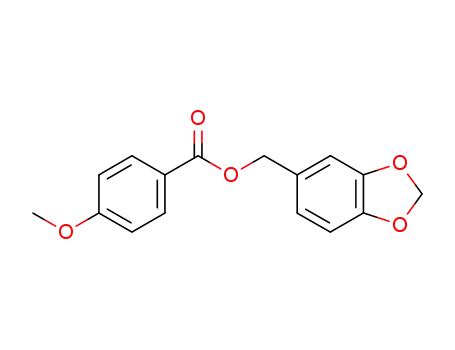
4-methoxy-benzoic acid piperonyl ester
-
5461-08-5
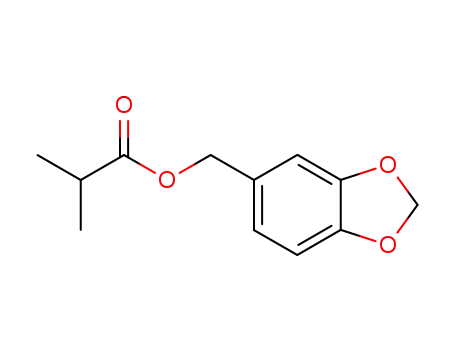
3,4-methylenedioxybenzyl isobutanoate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
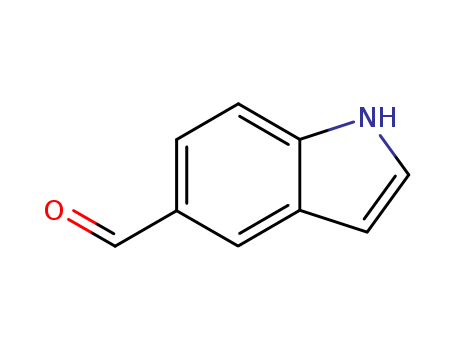
Indole-5-carboxaldehyde
CAS:1196-69-6
-
Piperonyl butoxide
CAS:51-03-6