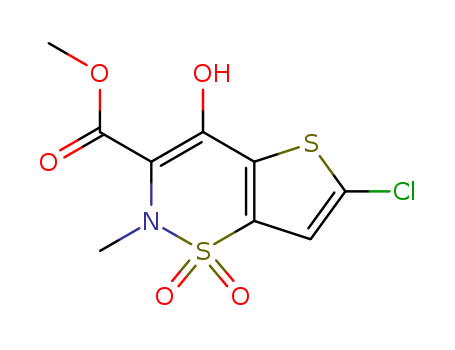
98623-50-8
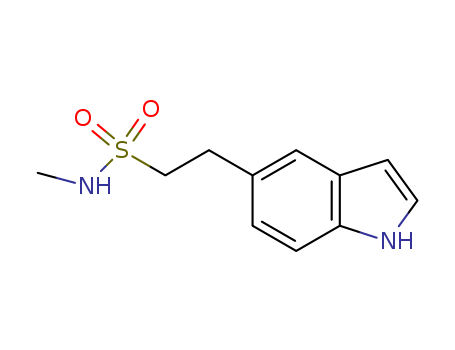
- Product Name:N-Methyl-1H-Indole-5-EthaneSulphonamide
- Molecular Formula:C11H14N2O2S
- Purity:99%
- Molecular Weight:238.31
Product Details
Manufacturer supply top purity N-Methyl-1H-Indole-5-EthaneSulphonamide 98623-50-8 with GMP standards
- Molecular Formula:C11H14N2O2S
- Molecular Weight:238.31
- Vapor Pressure:1.37E-08mmHg at 25°C
- Refractive Index:1.632
- Boiling Point:458.4 °C at 760 mmHg
- PKA:11.52±0.40(Predicted)
- Flash Point:231 °C
- PSA:70.34000
- Density:1.316g/cm3
- LogP:2.73130
N-Methyl-1H-Indole-5-EthaneSulphonamide(Cas 98623-50-8) Usage
InChI:InChI=1/C11H14N2O2S/c1-12-16(14,15)7-5-9-2-3-11-10(8-9)4-6-13-11/h2-4,6,8,12-13H,5,7H2,1H3
98623-50-8 Relevant articles
The photoredox-catalyzed hydrosulfamoylation of styrenes and its application in the novel synthesis of naratriptan
Chen, Miaomiao,Ding, Xin,Gao, Yongyue,He, Xingxing,Kang, Jin,Lu, Aidang,Wang, Qingmin,Wang, Ziwen,Zhang, Mingjun
, p. 9140 - 9143 (2021/09/14)
The hydrosulfamoylation of diverse aryl ...
An efficient synthetic protocol for the synthesis of 2-(1H-Indol-5-yl)-ethanesulfonic acid methylamide: A potential synthetic precursor for naratriptan and its novel 3-substituted derivatives
Behera, Ajaya Kumar,Majumdar, Poulomi,Mohanta, Prajna Parimita,Mishra, Sushanta Kumar
, p. 265 - 269 (2018/04/20)
Background: The 3-Substituted indoles ar...
A PROCESS FOR THE SYNTHESIS OF NARATRIPTAN
-
, (2011/04/13)
The present invention relates to a proce...
PROCESS FOR PREPARING INDOLE DERIVATIVES
-
Page/Page column 18-19, (2010/04/03)
The present invention provides a process...
98623-50-8 Process route
-
-
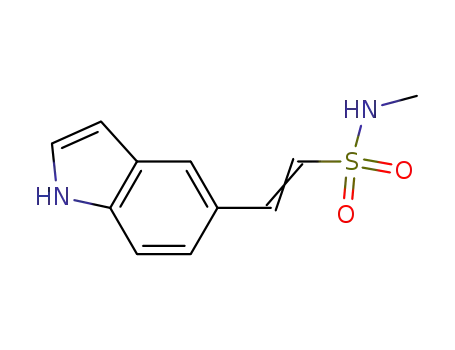
1305334-90-0
2-(1H-indol-5-yl)-N-methyl ethenesulfonamide

-
-
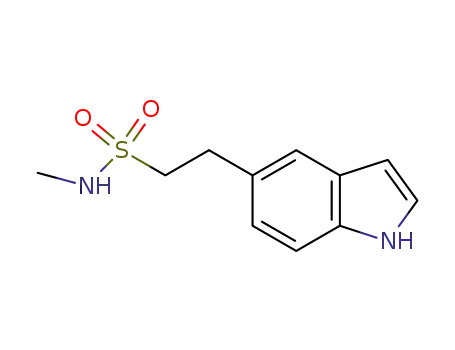
98623-50-8
N-Methyl-1H-indole-5-ethanesulphonamide
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol; ethyl acetate;
at 70 - 80 ℃;
for 6h;
Product distribution / selectivity;
|
90% |
-
![5-[2-(methylsulfamoyl)ethyl]-1H-indole-2-carboxylic acid](/upload/2024/12/84aeab80-0af4-4d28-9c70-0c2432eec55d.png)
-
1025797-51-6
5-[2-(methylsulfamoyl)ethyl]-1H-indole-2-carboxylic acid

-

-
98623-50-8
N-Methyl-1H-indole-5-ethanesulphonamide
| Conditions | Yield |
|---|---|
|
With
quinoline;
copper(I) oxide;
at 180 - 200 ℃;
for 6h;
|
76.66% |
|
copper chromite;
In
quinoline;
at 215 - 225 ℃;
Product distribution / selectivity;
|
65% |
|
copper chromite;
In
diphenylether;
at 25 - 225 ℃;
Product distribution / selectivity;
|
64% |
98623-50-8 Upstream products
-
1025797-51-6
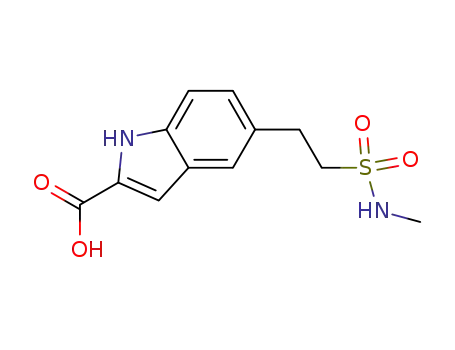
5-[2-(methylsulfamoyl)ethyl]-1H-indole-2-carboxylic acid
-
1190200-23-7
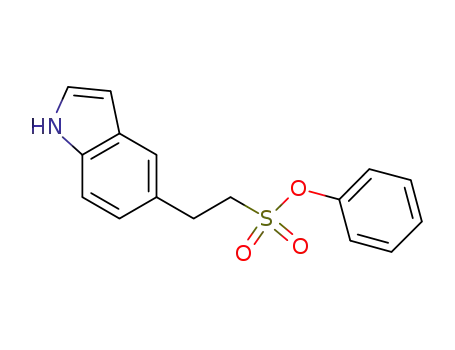
phenyl 2-(1H-indol-5-yl)ethanesulfonate
-
74-89-5
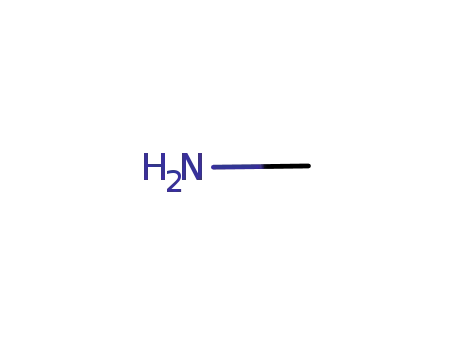
methylamine
-
1305334-90-0

2-(1H-indol-5-yl)-N-methyl ethenesulfonamide
98623-50-8 Downstream products
-
121679-20-7
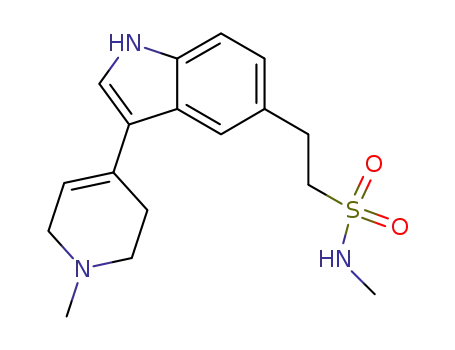
N-methyl-2-(3-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-5-yl)ethane-1-sulfonamide
-
143388-64-1
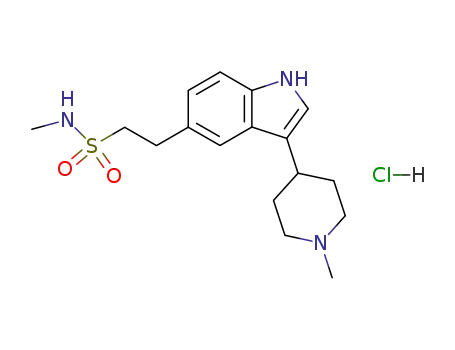
naratriptan hydrochloride
-
121679-13-8
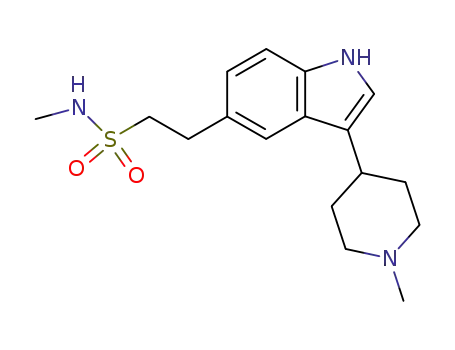
naratriptan
Relevant Products
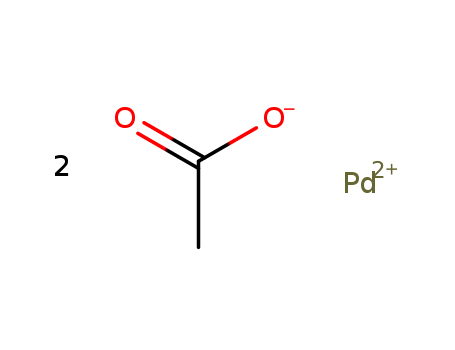
-
Palladium (II) Acetate
CAS:3375-31-3
-
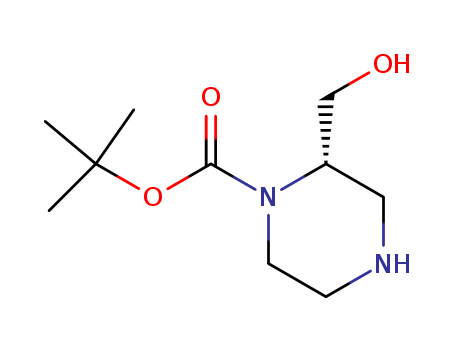
(S)-2-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
CAS:1030377-21-9