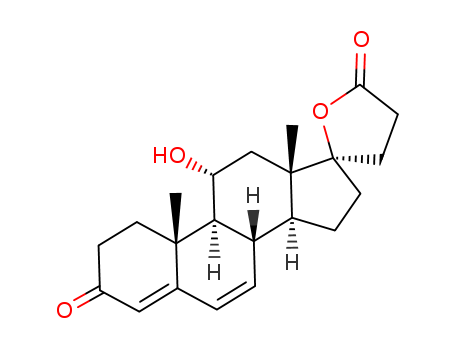
192704-56-6
- Product Name:11-a-Hydroxy canrenone methyl ester
- Molecular Formula:C24H32 O6
- Purity:99%
- Molecular Weight:416.514
Product Details
Manufacturer supply good quality 11-a-Hydroxy canrenone methyl ester 192704-56-6 with stock
- Molecular Formula:C24H32 O6
- Molecular Weight:416.514
- Boiling Point:604.5±55.0 °C(Predicted)
- PKA:14.40±0.70(Predicted)
- PSA:89.90000
- Density:1.27±0.1 g/cm3(Predicted)
- LogP:2.96400
192704-56-6 Relevant articles
A steroid compound derivative having, its preparation process and its use in the preparation of Eplerenone
-
, (2020/05/05)
The invention relates to a canrenone der...
A diastereoselective synthesis of 7α-nitromethyl steroid derivative and its use for an efficient synthesis of eplerenone
Zhang, Bin,Chen, Hongli,Tang, Huanyu,Feng, Huijin,Li, Yuanchao
, p. 1086 - 1091 (2012/11/13)
A novel and efficient method of stereose...
A chemobiological synthesis of eplerenone
Wuts, Peter G. M.,Anderson, Andrew M.,Ashford, Scott W.,Goble, Michael P.,White, Michael J.,Beck, Doris,Gilbert, Ivan,Hrab
, p. 418 - 422 (2008/09/17)
This paper will describe an approach to ...
PROCESS FOR PREPARING 7α-ALKOXYCARBONYL SUBSTITUTED STEROIDS
-
Page/Page column 68-69, (2008/06/13)
Processes are described for the conversi...
192704-56-6 Process route
-
-
209253-72-5
9α,11α-epoxy-17β-hydroxypregn-4-en-3-one-7α,21-dicarboxylic acid γ-lactone

-
-
74-88-4
methyl iodide

-
-
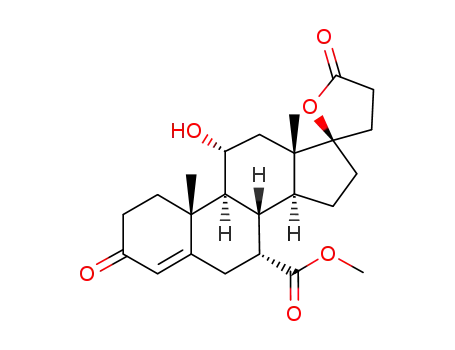
192704-56-6
11β,17β-dihydroxy-7α-methoxycarbonyl-pregna-4-en-3-one-21-carboxylic acid, γ-lactone
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetone;
at 0 - 20 ℃;
|
80% |
|
With
potassium carbonate;
at 0 - 20 ℃;
|
4 g |
-
-
C24H29NO5

-
-
865-33-8
potassium methanolate

-

-
192704-56-6
11β,17β-dihydroxy-7α-methoxycarbonyl-pregna-4-en-3-one-21-carboxylic acid, γ-lactone
| Conditions | Yield |
|---|---|
|
In
methanol;
at 62 - 115 ℃;
Product distribution / selectivity;
|
68% |
|
With
Methyl formate;
In
methanol;
at 60 - 115 ℃;
for 0.05 - 11.5h;
Product distribution / selectivity;
|
62.6% |
192704-56-6 Upstream products
-
192704-54-4
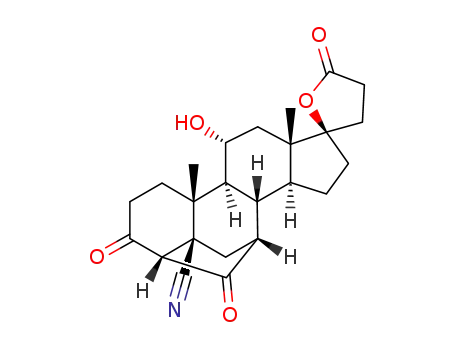
4'S(4'α),7'α-hexadecahydro-11'α-hydroxy-10'β,13'β-dimethyl-3',5,20'-trioxospiro[furan-2(3H),17'β-[4,7]metheno(17H)cyclopenta[a]phenanthrene]-5'β(2'H)-carbonitrile
-
124-41-4
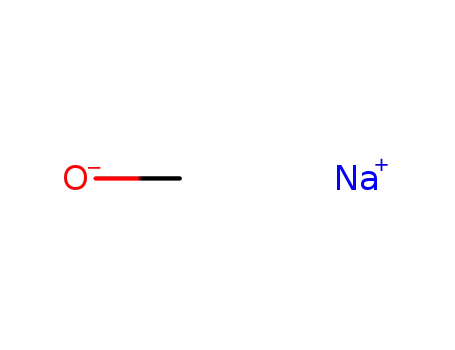
sodium methylate
-
67-56-1
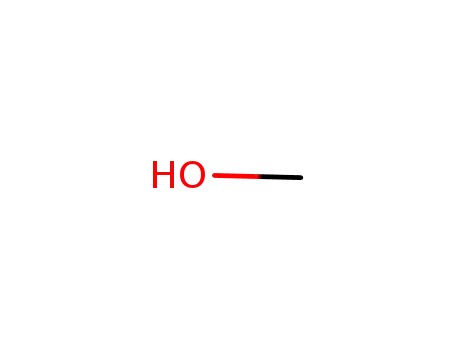
methanol
-
865-33-8

potassium methanolate
192704-56-6 Downstream products
-
192704-58-8
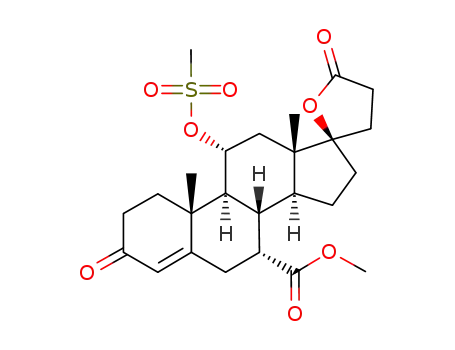
methyl hydrogen 17α-hydroxy-11α-(methylsulfonyl)oxy-3-oxopregn-4-ene-7α,21-dicarboxylate, γ-lactone
-
95716-70-4
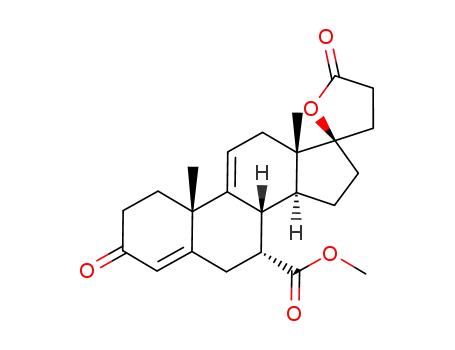
17α-pregna-4,9(11)-diene-7α,21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone-7-methyl ester
-
192704-70-4
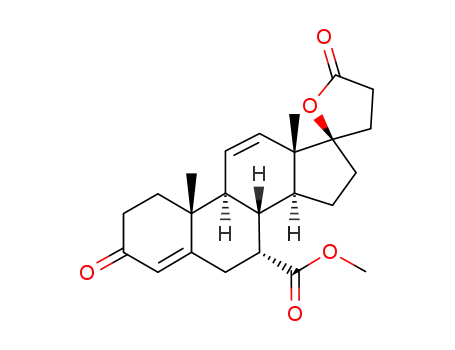
7-methyl hydrogen 17α-hydroxy-3-oxopregna-4,11-diene-7α,21-dicarboxylate, γ-lactone
-
107724-20-9
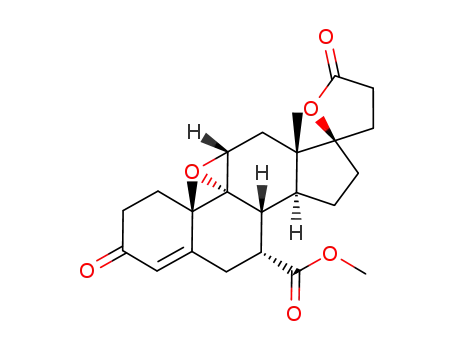
eplerenone
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
TRIFLOXYSTROBIN
CAS:141517-21-7
-
11-alpha-Hydroxycarvenone
CAS:192569-17-8