143900-44-1
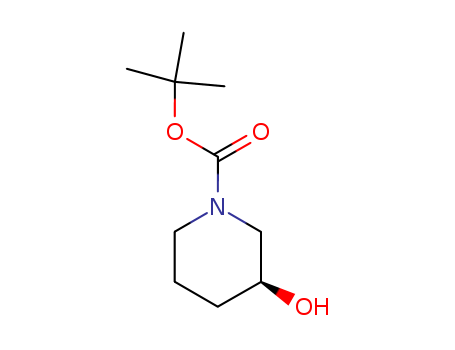
- Product Name:(S)-1-Boc-3-hydroxypiperidine
- Molecular Formula:C10H19NO3
- Purity:99%
- Molecular Weight:201.266
Product Details
pd_meltingpoint:34-40 °C
Factory Supply Industrial Grade (S)-1-Boc-3-hydroxypiperidine 143900-44-1 with Best Price
- Molecular Formula:C10H19NO3
- Molecular Weight:201.266
- Vapor Pressure:0.000198mmHg at 25°C
- Melting Point:34-40 °C
- Refractive Index:1.495
- Boiling Point:292.3 °C at 760 mmHg
- PKA:14.74±0.20(Predicted)
- Flash Point:130.6 °C
- PSA:49.77000
- Density:1.107 g/cm3
- LogP:1.31610
(S)-1-Boc-3-hydroxypiperidine(Cas 143900-44-1) Usage
InChI:InChI=1/C10H19NO3/c1-10(2,3)14-9(13)11-6-4-5-8(12)7-11/h8,12H,4-7H2,1-3H3/t8-/m0/s1
143900-44-1 Relevant articles
Efficient bioreductive production of (S)-N-Boc-3-hydroxypiperidine using ketoreductase ChKRED03
Xu, Guang-Peng,Wang, Hai-Bo,Wu, Zhong-Liu
, p. 881 - 885 (2016)
Ibrutinib is an anticancer drug targetin...
Characterization of a carbonyl reductase from Rhodococcus erythropolis WZ010 and its variant Y54F for asymmetric synthesis of (S)-N-Boc-3-hydroxypiperidine
Ying, Xiangxian,Zhang, Jie,Wang, Can,Huang, Meijuan,Ji, Yuting,Cheng, Feng,Yu, Meilan,Wang, Zhao,Ying, Meirong
, (2018)
The recombinant carbonyl reductase from ...
Efficient synthesis of Ibrutinib chiral intermediate in high space-time yield by recombinant E. coli co-expressing alcohol dehydrogenase and glucose dehydrogenase
Chen, Yitong,Ma, Baodi,Cao, Songshuang,Wu, Xiaomei,Xu, Yi
, p. 2325 - 2331 (2019)
The production of (S)-N-boc-3-hydroxy pi...
Engineering an Alcohol Dehydrogenase from Kluyveromyces polyspora for Efficient Synthesis of Ibrutinib Intermediate
Wu, Yanfei,Zhou, Jieyu,Ni, Jie,Zhu, Cheng,Sun, Zewen,Xu, Guochao,Ni, Ye
, (2021/02/26)
(S)-N-Boc-3-hydroxypiperidine [(S)-NBHP]...
Preparation method of N-tert-butyloxycarbonyl-3-piperidone and derivative thereof
-
Paragraph 0063; 0073-0078, (2020/07/21)
The invention discloses a preparation me...
Preparation method of (S)-1-tert-butyloxycarbonyl-3-hydroxypiperidine
-
, (2020/03/25)
The invention discloses a preparation me...
143900-44-1 Process route
-
-
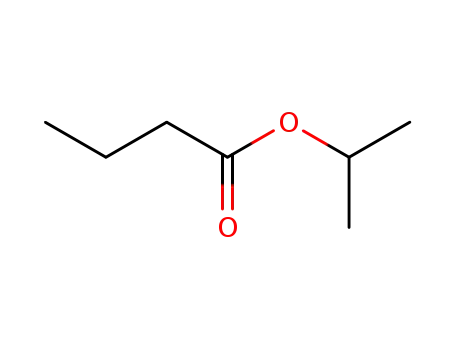
638-11-9
isopropyl butyrate

-
-
85275-45-2
N-tert-butoxycarbonyl-3-piperidinol

-
-
143900-43-0
tert-butyl (3R)-3-hydroxypiperidine-1-carboxylate

-
-
143900-44-1
tert-butyl (3S)-3-hydroxypiperidine-1-carboxylate

-
-
3-(S)-butyroxy-N-Boc-piperidine
| Conditions | Yield |
|---|---|
|
With
savinase enzyme; sodium carbonate;
In
toluene;
at 40 ℃;
for 20h;
Resolution of racemate;
Sealed tube;
Enzymatic reaction;
|
96.6 % ee 61.8 % ee |
-
-
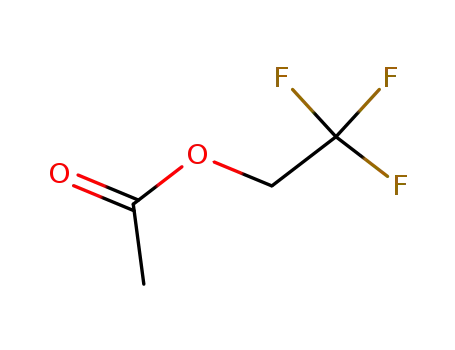
406-95-1
2,2,2-trifluoroethyl acetate

-

-
85275-45-2
N-tert-butoxycarbonyl-3-piperidinol

-
-
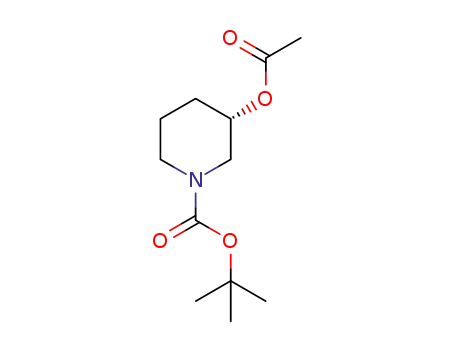
(S)-1-tert-butoxycarbonyl-3-acetoxypiperidine

-

-
143900-43-0
tert-butyl (3R)-3-hydroxypiperidine-1-carboxylate

-

-
143900-44-1
tert-butyl (3S)-3-hydroxypiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
savinase enzyme; sodium carbonate;
In
toluene;
at 40 ℃;
for 20h;
Resolution of racemate;
Sealed tube;
Enzymatic reaction;
|
99.1 % ee 61.8 % ee |
143900-44-1 Upstream products
-
108-05-4
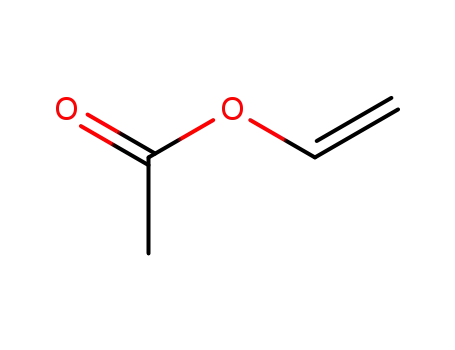
vinyl acetate
-
85275-45-2

N-tert-butoxycarbonyl-3-piperidinol
-
24424-99-5
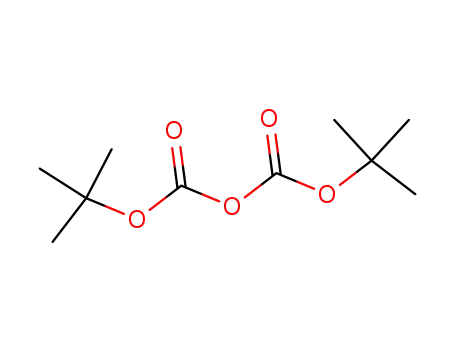
di-tert-butyl dicarbonate
-
475058-41-4
(S)-3-hydroxypiperidine hydrochloride
143900-44-1 Downstream products
-
940890-90-4
(S)-3-methanesulfonyloxy-piperidine-1-carboxylic acid tert-butyl ester
-
316353-98-7
(S)-N-methylpiperidin-3-yl butyrate
-
1022150-11-3
(3R)‐4‐amino‐3‐(4-phenoxyphenyl)‐1‐(1‐tert-butoxycarbonylpiperidine-3-yl)-1H-pyrazolo[3,4-d]pyrimidine
-
1073136-01-2
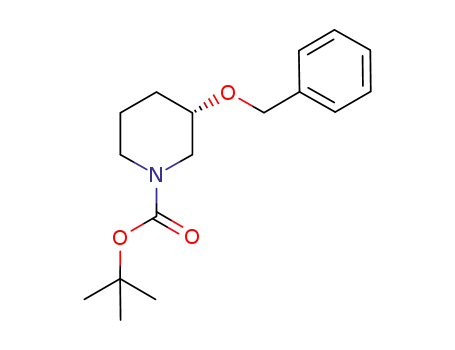
tert-butyl (+)-(3S)-3-(benzyloxy)piperidine carbamate
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
KRESOXIM-METHYL
CAS:143390-89-0
-
TRIFLOXYSTROBIN
CAS:141517-21-7