976-71-6
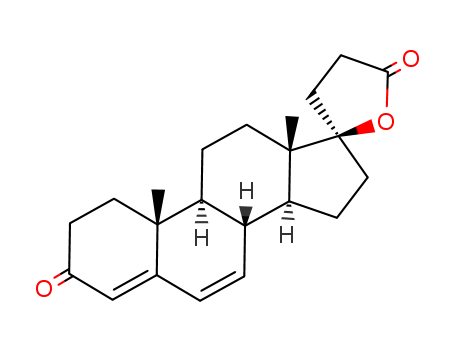
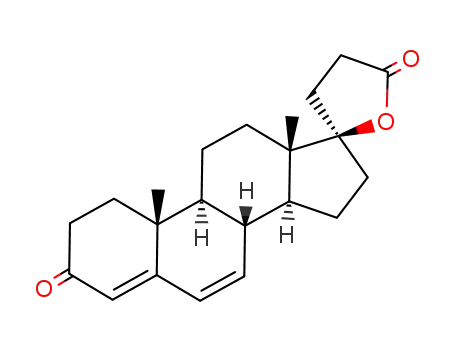
- Product Name:Canrenone
- Molecular Formula:C22H28O3
- Purity:99%
- Molecular Weight:340.463
Product Details
pd_meltingpoint:158-160 °C
Factory Sells Best Quality Canrenone 976-71-6 with stock
- Molecular Formula:C22H28O3
- Molecular Weight:340.463
- Vapor Pressure:8.95E-12mmHg at 25°C
- Melting Point:158-160 °C
- Refractive Index:1.581
- Boiling Point:541.1 °C at 760 mmHg
- Flash Point:237.6 °C
- PSA:43.37000
- Density:1.19 g/cm3
- LogP:4.37010
Canrenone(Cas 976-71-6) Usage
|
World Health Organization (WHO) |
Canrenone, which has aldosterone antagonist activity, is a major metabolite of spironolactone and the major metabolite of potassium canrenoate. See WHO comments for potassium canrenoate and spironolactone. |
|
Flammability and Explosibility |
Nonflammable |
|
Biological Activity |
Mineralocorticoid receptor antagonist. Active metabolite of spironolactone ((7a,17a)-7-(Acetylthio)-17-hydroxy-3-oxopregn-4-ene-21-carboxylic acid g-lactone ). |
|
Biochem/physiol Actions |
Canrenone is a mineralocorticoid (aldosterone) inhibitor. |
|
Brand name |
Luvion. |
InChI:InChI=1/C22H28O3/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21)12-8-19(24)25-22/h3-4,13,16-18H,5-12H2,1-2H3/t16?,17?,18?,20-,21-,22+/m0/s1
976-71-6 Relevant articles
Preformulation studies of spironolactone: Effect of pH, two buffer species, ionic strength, and temperature on stability
Pramar,Gupta
, p. 551 - 553 (1991)
Using a stability-indicating HPLC assay ...
The nitration of canrenone with acetic anhydride/nitric acid
Megges, Rudolf,Weiland, Juergen,Undeutsch, Bernd,Buechting, Horst,Schoen, Rudolf
, p. 762 - 766 (1997)
3-Oxo-17α-pregna-4,6-diene-21,17-carbola...
Synthesis and reactions of 2-methylene-canrenone
Gorlitzer,Moormann,Pollow,Schaffrath
, p. 149 - 155 (1995)
Starting from the Mannich salt 1 of the ...
Method for preparing spirolactone intermediate canrenone
-
Paragraph 0019-0033, (2021/10/20)
The invention provides a method for prep...
One-Pot γ-Lactonization of Homopropargyl Alcohols via Intramolecular Ketene Trapping
Yamane, Daichi,Tanaka, Haruna,Hirata, Akihiro,Tamura, Yumiko,Takahashi, Daichi,Takahashi, Yusuke,Nagamitsu, Tohru,Ohtawa, Masaki
, p. 2831 - 2835 (2021/05/05)
A one-pot γ-lactonization of homoproparg...
Synthesis method of canrenone
-
Paragraph 0047-0190, (2021/10/05)
The invention provides a synthesis metho...
Synthesis process of steroid compound, canrenone and spirolactone
-
, (2020/11/23)
The invention relates to the technical f...
976-71-6 Process route
-
-
C25H32O5

-
-
976-71-6
canrenone
| Conditions | Yield |
|---|---|
|
With
poly(4-vinylpyridine);
In
2-methyltetrahydrofuran; water; N,N-dimethyl-formamide;
at 65 ℃;
for 6h;
Solvent;
Temperature;
Reagent/catalyst;
|
85.82% |
|
With
acetic acid;
In
water; toluene;
at 0 - 100 ℃;
for 24h;
pH=7.1;
Temperature;
pH-value;
Autoclave;
Inert atmosphere;
|
33.1 g |
-
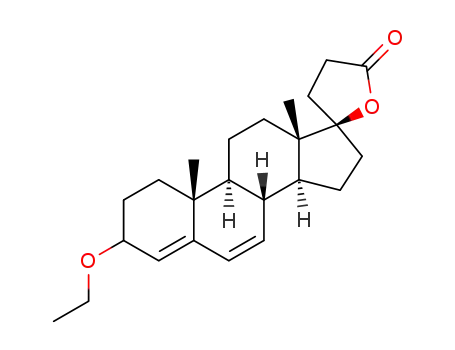
-
17β-hydroxy-3-ethoxy-17α-pregn-3,5-diene-21-carboxylic acid-γ-lactone

-

-
976-71-6
canrenone
| Conditions | Yield |
|---|---|
|
17β-hydroxy-3-ethoxy-17α-pregn-3,5-diene-21-carboxylic acid-γ-lactone;
With
chloranil;
In
methanol; dichloromethane; water;
at 20 ℃;
for 1h;
With
sodium thiosulfate;
In
methanol; water;
at 20 ℃;
for 1h;
|
83% |
976-71-6 Upstream products
-
976-70-5
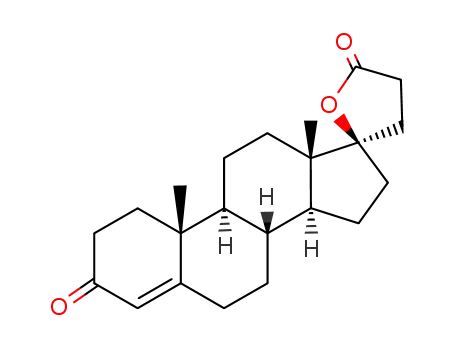
3-oxopregn-4-ene-21,17α-carbolactone
-
13934-61-7
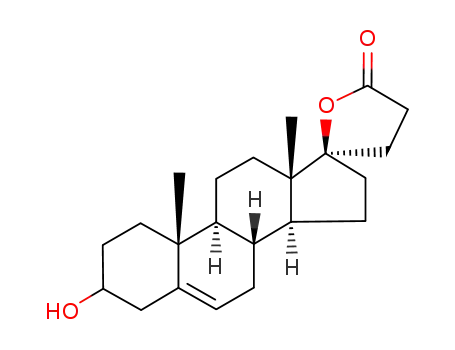
C22H32O3
-
52-01-7
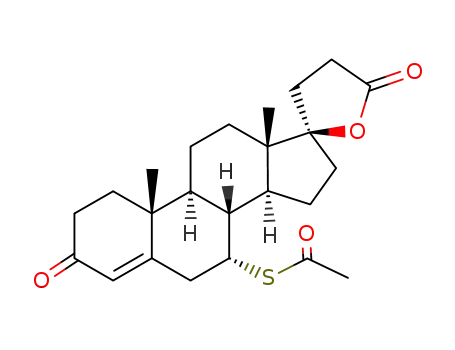
SPIRONOLACTONE
-
434-03-7
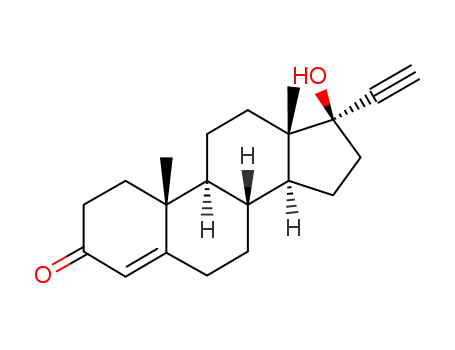
levonorgestrel
976-71-6 Downstream products
-
52-01-7

SPIRONOLACTONE
-
66175-65-3
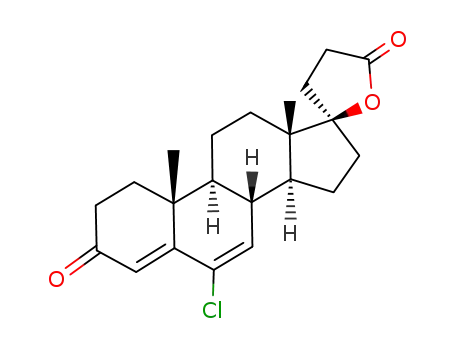
C22H27ClO3
-
110078-67-6
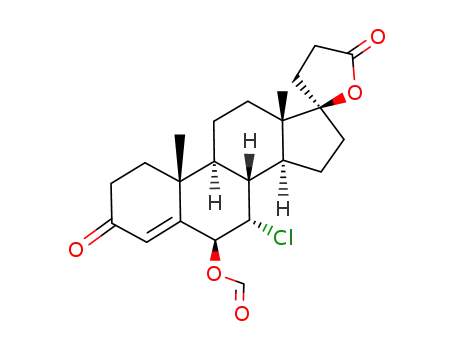
7α-Chlor-6β-(formyloxy)-17β-hydroxy-3-oxo-4,6-pregnadien-21-carbonsaeure-γ-lacton
-
106112-12-3
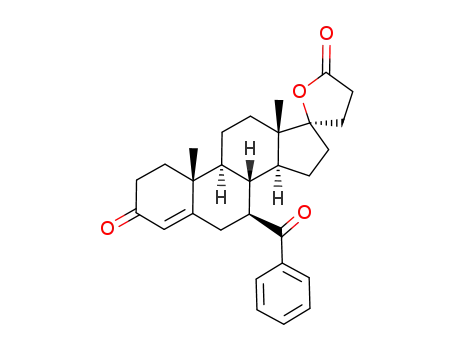
C29H34O4
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
2,6-Dichlorobenzyl chloride
CAS:2014-83-7
-
KRESOXIM-METHYL
CAS:143390-89-0