349-88-2
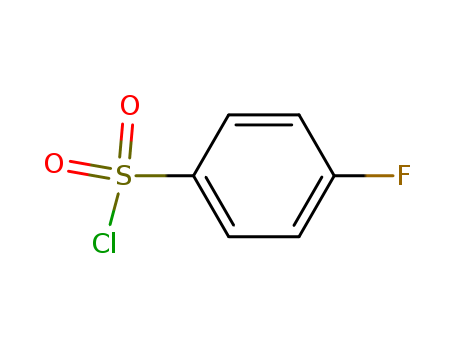
- Product Name:4-Fluorobenzenesulfonyl chloride
- Molecular Formula:C6H4ClFO2S
- Purity:99%
- Molecular Weight:194.614
Product Details
pd_meltingpoint:29-31 °C(lit.)
Appearance:white to light brown crystalline low melting mass
Manufacturer supply high quality 4-Fluorobenzenesulfonyl chloride 349-88-2 with ISO standards
- Molecular Formula:C6H4ClFO2S
- Molecular Weight:194.614
- Appearance/Colour:white to light brown crystalline low melting mass
- Melting Point:29-31 °C(lit.)
- Boiling Point:263.2 °C at 760 mmHg
- Flash Point:113 °C
- PSA:42.52000
- Density:1.493 g/cm3
- LogP:2.83400
4-Fluorobenzenesulfonyl chloride(Cas 349-88-2) Usage
InChI:InChI=1/C6H5F.Cl2O2S/c7-6-4-2-1-3-5-6;1-5(2,3)4/h1-5H;
349-88-2 Relevant articles
Chromoselective Synthesis of Sulfonyl Chlorides and Sulfonamides with Potassium Poly(heptazine imide) Photocatalyst
Antonietti, Markus,Guldi, Dirk M.,Markushyna, Yevheniia,Savateev, Aleksandr,Schü?lbauer, Christoph M.,Ullrich, Tobias
supporting information, p. 20543 - 20550 (2021/08/12)
Among external stimuli used to promote a...
Facile synthesis of sulfonyl chlorides/bromides from sulfonyl hydrazides
Chen, Rongxiang,Xu, Shaohong,Shen, Fumin,Xu, Canran,Wang, Kaikai,Wang, Zhanyong,Liu, Lantao
, (2021/09/20)
A simple and rapid method for efficient ...
Synthesis, biological evaluation, and enzyme assay of some 5-N-substituted-2-N-(arylsulphonyl)-L(+)glutamines as potential anticancer agents
Jha, Tarun,Samanta, Soma,Halder, Amit Kumar,Adhikari, Nilanjan,Abdul Amin,Sanyal, Arpita,Mukherjee, Tanmoy
, p. 1259 - 1264 (2020/12/04)
Thirty 5-N-substituted-2-N-(arylsulphony...
Preparation method for symmetric diaryl disulfide
-
Paragraph 0048; 0049; 0050; 0051, (2019/04/17)
The invention discloses a preparation me...
349-88-2 Process route
-
-
2266-41-3
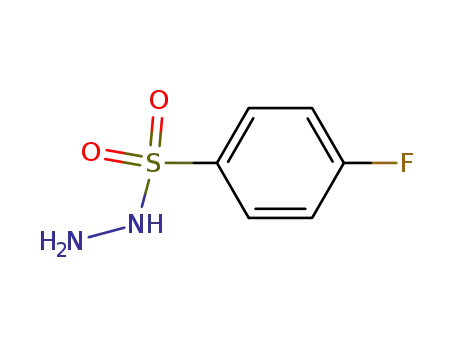
4-fluorobenzenesulfonylhydrazide

-
-
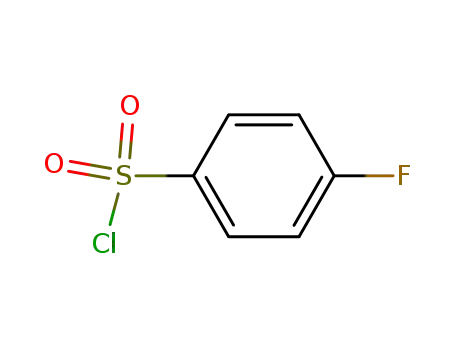
349-88-2
4-Fluorobenzenesulfonyl chloride
| Conditions | Yield |
|---|---|
|
With
N-chloro-succinimide;
In
acetonitrile;
at 20 ℃;
for 2h;
|
96% |
-
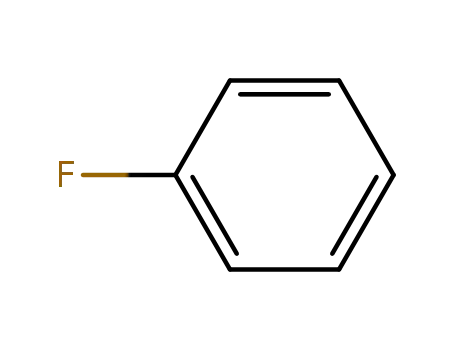
-
462-06-6
fluorobenzene

-

-
349-88-2
4-Fluorobenzenesulfonyl chloride
| Conditions | Yield |
|---|---|
|
With
chlorosulfonic acid;
|
95% |
|
With
chlorosulfonic acid;
for 1h;
|
90% |
|
With
chlorosulfonic acid;
at 0 - 5 ℃;
|
80.44% |
|
With
chlorosulfonic acid;
at 0 - 5 ℃;
|
76.27% |
|
With
chlorosulfonic acid;
|
28% |
|
With
chlorosulphuric acid; chloroform;
|
|
|
With
chlorosulfonic acid;
|
|
|
With
chlorosulphuric acid;
1) 0 deg C; 2) room temperature, 2 h;
|
|
|
With
chlorosulfonic acid;
|
|
|
With
chlorosulfonic acid;
at 0 - 5 ℃;
for 4.5h;
|
|
|
fluorobenzene;
With
chlorosulfonic acid;
at 150 ℃;
for 2.5h;
With
thionyl chloride;
at 150 ℃;
for 3h;
|
|
|
With
chlorosulfonic acid;
for 6h;
Reflux;
|
|
|
fluorobenzene;
With
chlorosulfonic acid;
In
ethanol;
at 5 ℃;
for 6h;
With
thionyl chloride;
In
ethanol;
at 75 ℃;
for 5h;
|
349-88-2 Upstream products
-
462-06-6
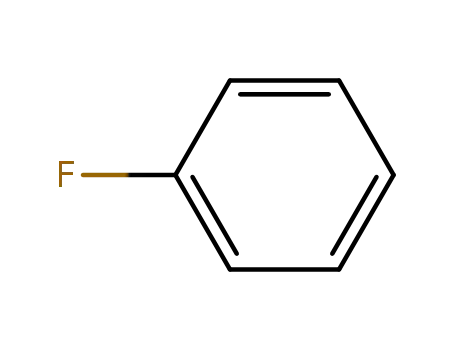
fluorobenzene
-
371-42-6
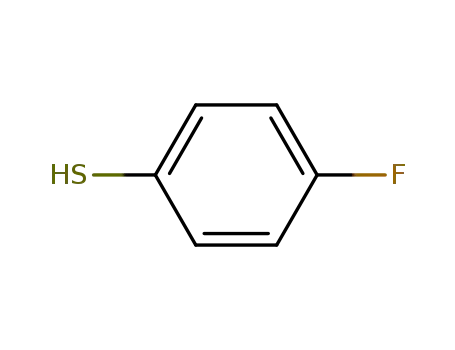
4-Fluorothiophenol
-
368-85-4
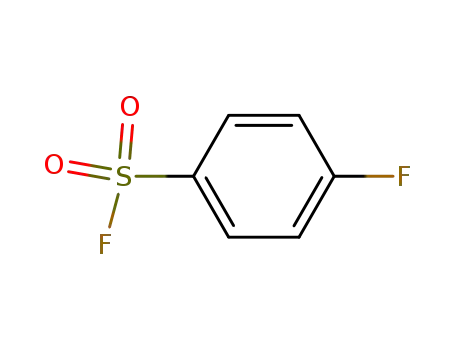
4-fluorobenzenesulfonyl fluoride
-
371-40-4
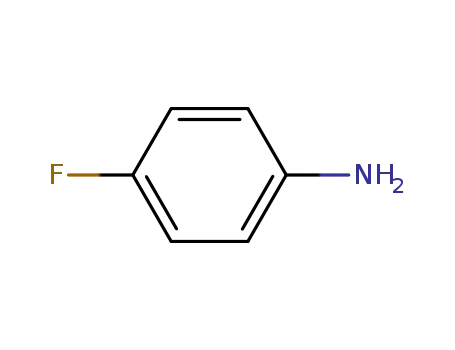
4-fluoroaniline
349-88-2 Downstream products
-
312-32-3
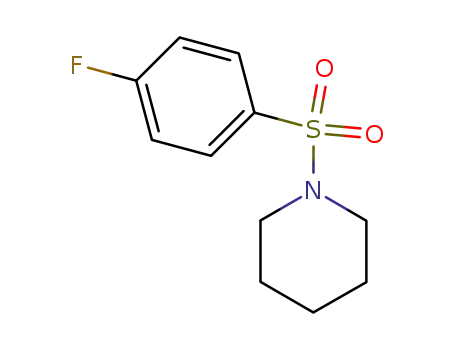
1-[(4-Fluorophenyl)sulfonyl]piperidine
-
383-23-3
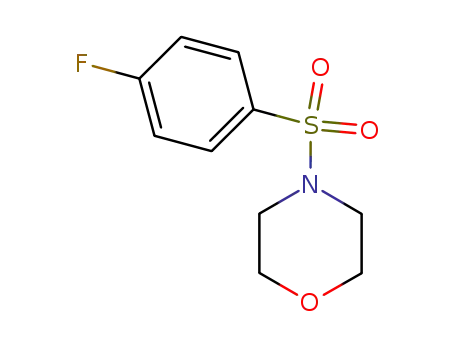
4-[(4-fluorophenyl)sulfonyl]morpholine
-
383-46-0
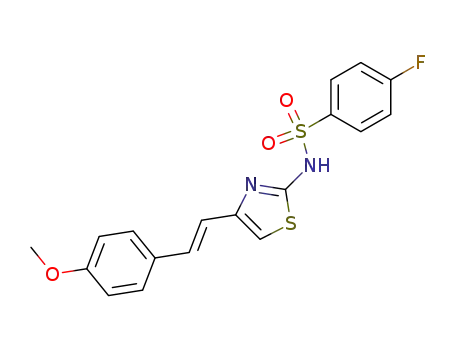
4-fluoro-benzenesulfonic acid-[4-(4-methoxy-trans-styryl)-thiazol-2-ylamide]
-
339-37-7
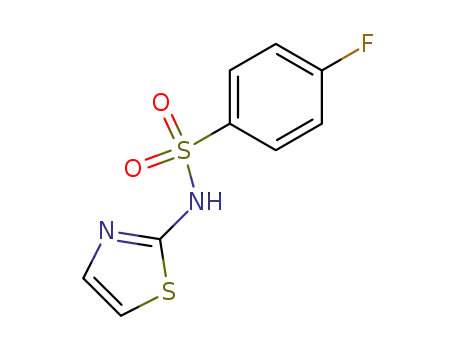
4-fluoro-benzenesulfonic acid thiazol-2-ylamide
Relevant Products
-
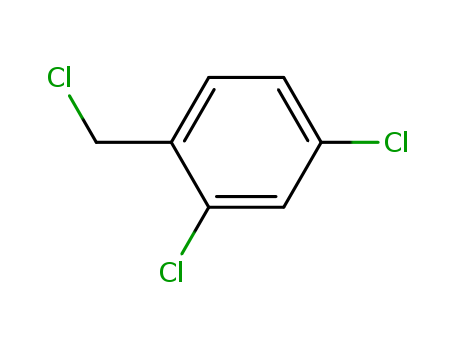
2,4-Dichlorobenzyl chloride
CAS:94-99-5
-
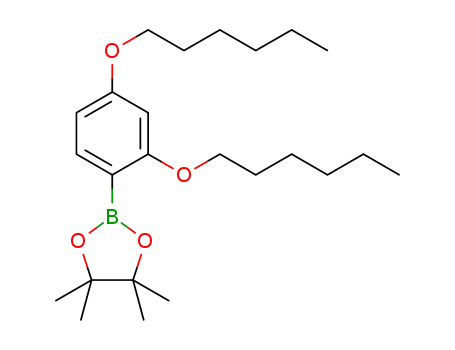
2,4-Bis(hexyloxy)phenylboronic acid pinacol ester
CAS:1391734-70-5