104-63-2
- Product Name:N-Benzylethanolamine
- Molecular Formula:C9H13NO
- Purity:99%
- Molecular Weight:151.208
Product Details
pd_meltingpoint:-30℃
Appearance:light yellow viscid transparent liquid
Manufacturer supply good quality N-Benzylethanolamine 104-63-2 with stock
- Molecular Formula:C9H13NO
- Molecular Weight:151.208
- Appearance/Colour:light yellow viscid transparent liquid
- Vapor Pressure:0.00134mmHg at 25°C
- Melting Point:-30℃
- Refractive Index:n20/D 1.543(lit.)
- Boiling Point:285.1 °C at 760 mmHg
- PKA:14.59±0.10(Predicted)
- Flash Point:131.8 °C
- PSA:32.26000
- Density:1.065 g/cm3
- LogP:1.15940
N-Benzylethanolamine(Cas 104-63-2) Usage
InChI:InChI=1/C9H13NO/c11-7-6-10-8-9-4-2-1-3-5-9/h1-5,10-11H,6-8H2/p+1
104-63-2 Relevant articles
A One-pot Synthesis of Nitrohydroxylated Pyrrolidine and Piperidine Ring Sytems by Tandem Michael-Henry Reaction
Barco, Achille,Benetti, Simonetta,Risi, Carmela De,Pollini, Gian P.,Romagnoli, Romeo,Zanirato, Vinicio
, p. 9293 - 9296 (1994)
Nitrohydroxylated pyrrolidine and piperi...
Discovery of benzimidazole analogs as a novel interleukin-5 inhibitors
Boggu, Pulla Reddy,Kim, Youngsoo,Jung, Sang-Hun
, (2019)
A series of novel hydroxyethylaminomethy...
Synthesis of β-amino alcohols from aromatic amines and alkylene carbonates using Na-Y zeolite catalyst
Shivarkar, Anandkumar B.,Gupte, Sunil P.,Chaudhari, Raghunath V.
, p. 1374 - 1378 (2006)
A simple, efficient, and environmentally...
AgI/TMG-Promoted Cascade Reaction of Propargyl Alcohols, Carbon Dioxide, and 2-Aminoethanols to 2-Oxazolidinones
Li, Xue-Dong,Song, Qing-Wen,Lang, Xian-Dong,Chang, Yao,He, Liang-Nian
, p. 3182 - 3188 (2017)
Chemical valorization of CO2 to access v...
Charge neutral rhenium tricarbonyl complexes of tridentate N-heterocyclic carbene ligands that bind to amyloid plaques of Alzheimer's disease
Barnard, Peter J.,Donnelly, Paul S.,McLean, Catriona A.,Noor, Asif,Wiratpruk, Nuchareenat
, p. 4559 - 4569 (2020)
Two tridentate ligand systems bearing N-...
Convenient methods for the hydrolysis of oxazolidinones to vicinal aminoalcohols
Katz, Steven J,Bergmeier, Stephen C
, p. 557 - 559 (2002)
We have developed two convenient methods...
A Convenient Reduction of Amino Acids and Their Derivatives
McKennon, Marc J.,Meyers, A. I.,Drauz, Karlheinz,Schwarm, Michael
, p. 3568 - 3571 (1993)
-
Introduction of the 4,4,4-Trifluorobut-2-ene Chain Exploiting a Regioselective Tsuji-Trost Reaction Catalyzed by Palladium Nanoparticles
Hemelaere, Rémy,Desroches, Justine,Paquin, Jean-Fran?ois
, p. 1770 - 1773 (2015)
A palladium-nanoparticle-catalyzed Tsuji...
Ionic liquid-promoted three-component domino reaction of propargyl alcohols, carbon dioxide and 2-aminoethanols: A thermodynamically favorable synthesis of 2-oxazolidinones
Xia, Shumei,Song, Yu,Li, Xuedong,Li, Hongru,He, Liang-Nian
, (2018)
To circumvent the thermodynamic limitati...
Selective O-benzylation of aminoalkanols
Hu,Cassady
, p. 907 - 913 (1995)
A simple and one-step method has been de...
Optimization of gefitinib analogues with potent anticancer activity
Yin, Kai-Hao,Hsieh, Yi-Han,Sulake, Rohidas S.,Wang, Su-Pei,Chao, Jui-I.,Chen, Chinpiao
, p. 5247 - 5250 (2014)
The interactions of gefitinib (Iressa) i...
Nickel-Catalyzed Regio- And Stereospecific C-H Coupling of Benzamides with Aziridines
Hirano, Koji,Miura, Masahiro,Xu, Shibo
supporting information, p. 5471 - 5475 (2021/07/26)
A nickel-catalyzed C-H coupling of 8-ami...
Radiosynthesis and evaluation of 18F-labeled dopamine D4-receptor ligands
Willmann, Michael,Ermert, Johannes,Prante, Olaf,Hübner, Harald,Gmeiner, Peter,Neumaier, Bernd
, p. 43 - 52 (2020/08/03)
Introduction: The dopamine D4 receptor (...
BTK Inhibitors and uses thereof
-
Paragraph 1107-1112, (2020/05/02)
The invention discloses a bruton's tyros...
104-63-2 Process route
-
-
17886-25-8
1-benzyl-4,5-dihydro-1H-[1,2,3]triazole

-
-
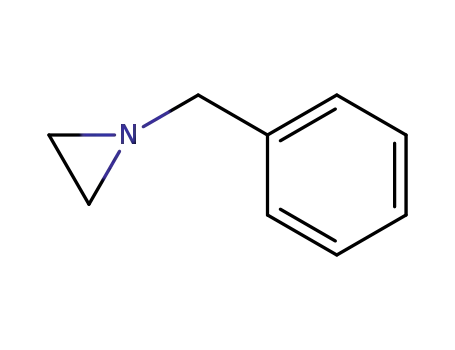
1074-42-6
1-benzylaziridine

-
-
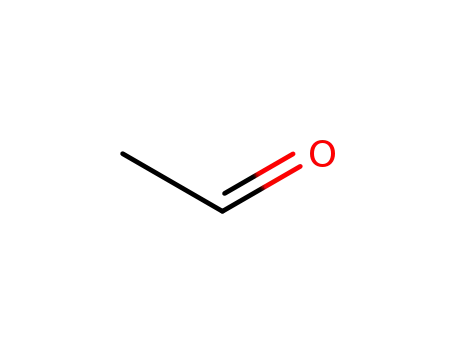
75-07-0,9002-91-9
acetaldehyde

-
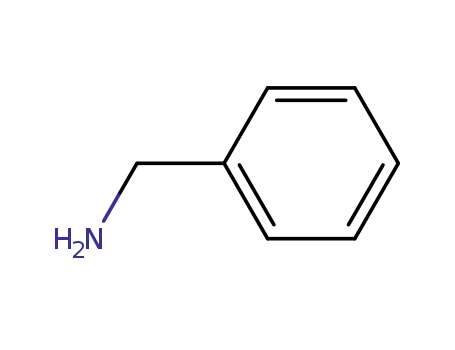
-
100-46-9
benzylamine

-
-
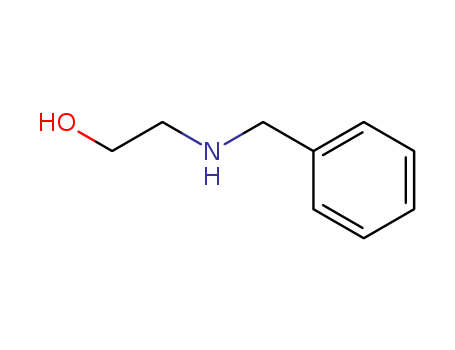
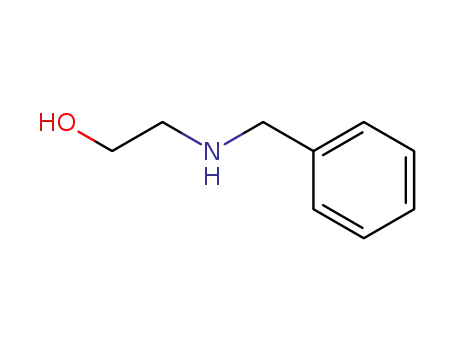
104-63-2
N-Benzylethanolamine
| Conditions | Yield |
|---|---|
|
With
water;
at 25 ℃;
Rate constant;
Product distribution;
Mechanism;
pH 10.75 (lysine buffer); other solvent, other pH (other buffer concentration, other buffer), H/D isotope effect;
|
-
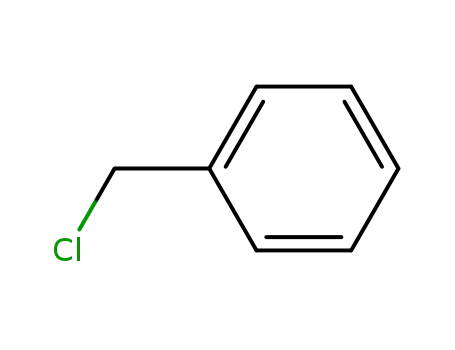
-
100-44-7
benzyl chloride

-
-
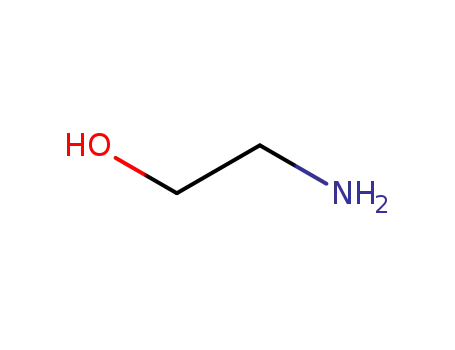
141-43-5
ethanolamine

-
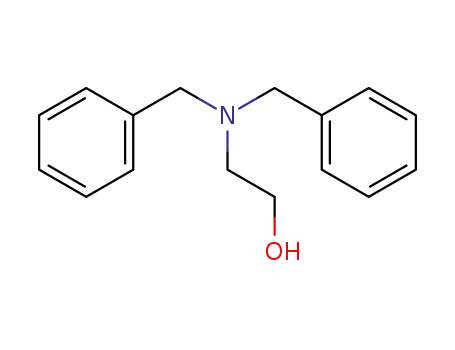
-
101-06-4
N,N-dibenzyl-2-aminoethanol

-

-
104-63-2
N-Benzylethanolamine
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; ethanol;
|
104-63-2 Upstream products
-
75-21-8
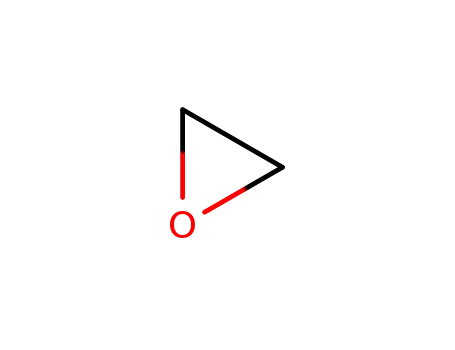
oxirane
-
100-46-9
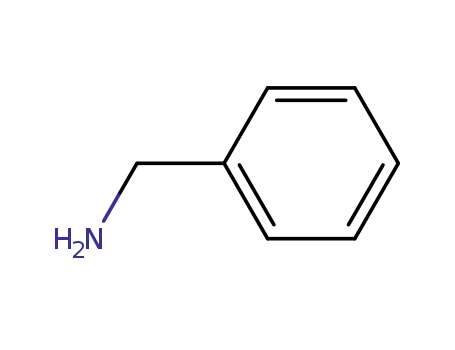
benzylamine
-
495-69-2
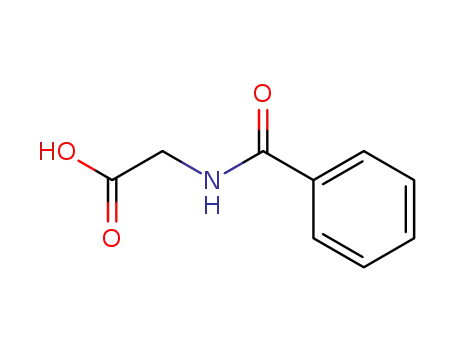
Hippuric Acid
-
1205-08-9
methyl hippurate
104-63-2 Downstream products
-
385810-98-0
N-benzyl-2-(2-pyridyloxy)ethylamine
-
872298-31-2
2-(N-benzyl-pyridin-2-yl-amino)ethanol
-
100717-66-6
2-(benzyl-[2]thienylmethyl-amino)-ethanol
-
114461-89-1
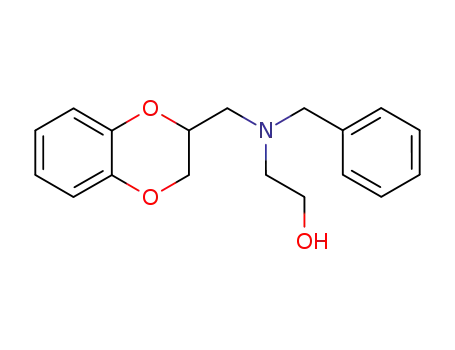
2-[benzyl-(2,3-dihydro-benzo[1,4]dioxin-2-ylmethyl)-amino]-ethanol
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
Dodecyltrimethylammonium chloride
CAS:112-00-5
-
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)
CAS:6674-22-2