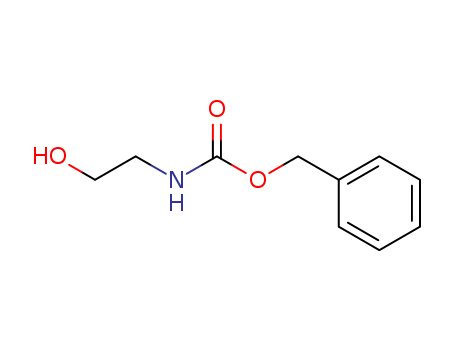
77987-49-6
- Product Name:BENZYL N-(2-HYDROXYETHYL)CARBAMATE
- Molecular Formula:C10H13NO3
- Purity:99%
- Molecular Weight:195.218
Product Details
pd_meltingpoint:50-60 °C
Manufacturer Sells Best Quality BENZYL N-(2-HYDROXYETHYL)CARBAMATE 77987-49-6 with stock
- Molecular Formula:C10H13NO3
- Molecular Weight:195.218
- Vapor Pressure:3.28E-06mmHg at 25°C
- Melting Point:50-60 °C
- Refractive Index:1.542
- Boiling Point:372.5 °C at 760 mmHg
- PKA:11.80±0.46(Predicted)
- Flash Point:179.1 °C
- PSA:58.56000
- Density:1.182 g/cm3
- LogP:1.29600
BENZYL N-(2-HYDROXYETHYL)CARBAMATE(Cas 77987-49-6) Usage
InChI:InChI=1/C10H13NO3/c12-7-6-11-10(13)14-8-9-4-2-1-3-5-9/h1-5,12H,6-8H2,(H,11,13)
77987-49-6 Relevant articles
Preparation method of N-(2-aminoethyl) morpholine
-
Paragraph 0062-0064; 0073-0075, (2021/04/21)
The invention provides a preparation met...
Oxyenamides as Versatile Building Blocks for a Highly Stereoselective One-Pot Synthesis of the 1,3-Diamino-2-ol-Scaffold Containing Three Continuous Stereocenters
Bolte, Michael,Grimmer, Jennifer,Kelm, Harald,Kramer, Philipp,Krieg, Sara-Cathrin,Manolikakes, Georg
supporting information, p. 23667 - 23671 (2021/09/30)
A highly diastereoselective one-pot synt...
Interplay between a Foldamer Helix and a Macrocycle in a Foldarotaxane Architecture
Gauthier, Maxime,Koehler, Victor,Clavel, Caroline,Kauffmann, Brice,Huc, Ivan,Ferrand, Yann,Coutrot, Frédéric
supporting information, p. 8380 - 8384 (2021/03/16)
The design and synthesis of a novel rota...
PROCESS OF PREPARING ARACHIDONOYLETHANOLAMINE ANALOGUES
-
Page/Page column 25, (2021/05/29)
The present application provides new pro...
77987-49-6 Process route
-
-
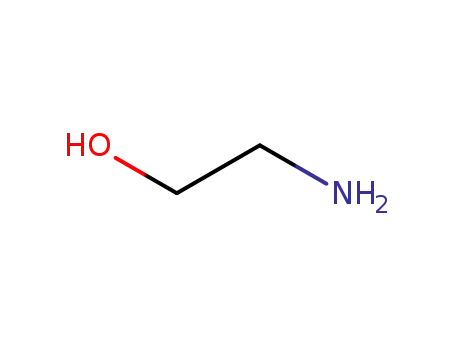
141-43-5
ethanolamine

-
-
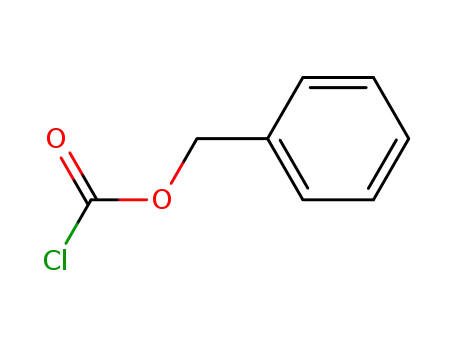
501-53-1,94274-21-2
benzyl chloroformate

-
-
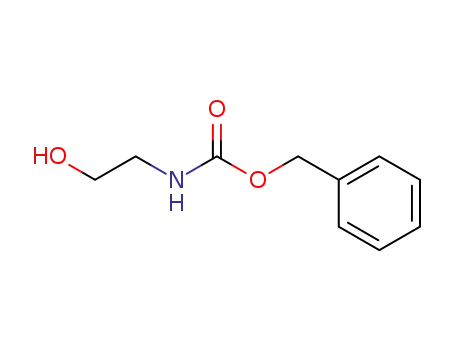
77987-49-6
benzyl 2-hydroxyethylcarbamate
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 1h;
|
100% |
|
In
benzene;
for 0.5h;
Ambient temperature;
|
95% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 6h;
Large scale;
|
95.3% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
|
93% |
|
With
sulfuric acid; silica gel;
at 20 ℃;
for 0.166667h;
|
92% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
1) 0 deg C -> r.t., 1 h, 2) r.t., 4 h;
|
90% |
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide; acetonitrile;
for 1h;
Ambient temperature;
|
90% |
|
With
sodium hydroxide;
In
dichloromethane;
at 20 ℃;
for 2h;
|
90% |
|
With
sodium hydroxide;
In
dichloromethane; water;
at 20 ℃;
|
90% |
|
In
PEG-600;
at 20 ℃;
for 0.0833333h;
chemoselective reaction;
|
89% |
|
With
dimethylbromosulphonium bromide;
In
toluene;
at 20 ℃;
for 0.2h;
chemoselective reaction;
|
87% |
|
With
triethylamine;
In
dichloromethane;
|
86% |
|
With
triethylamine;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
85.2% |
|
With
trimethylamine;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1h;
|
85.2% |
|
With
sodium carbonate;
at 0 - 8 ℃;
for 3h;
|
83% |
|
With
cetyltrimethylammonim bromide;
In
water;
at 30 ℃;
for 0.166667h;
|
83% |
|
With
sodium hydroxide;
In
water;
1.) 0 deg C, 70 min; 2.) RT, 30 min;
|
81% |
|
With
dmap; TEA;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 12h;
|
80% |
|
With
sodium carbonate;
In
tetrahydrofuran; water;
at 22 ℃;
for 24h;
|
78.3% |
|
With
triethylamine;
In
dichloromethane;
at 10 - 25 ℃;
for 5h;
|
78% |
|
With
triethylamine;
In
dichloromethane; water;
|
77% |
|
With
triethylamine;
In
dichloromethane; water;
|
77% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 20 ℃;
for 1h;
|
76% |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 2h;
|
71% |
|
at 0 ℃;
for 1h;
|
70% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 1h;
|
70% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 1h;
|
70% |
|
ethanolamine;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 0.0833333h;
Inert atmosphere;
benzyl chloroformate;
In
water;
at 20 ℃;
for 2h;
Inert atmosphere;
|
68% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
Schlenk technique;
|
67% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
63% |
|
With
triethylamine;
In
dichloromethane;
|
63% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
61% |
|
With
sodium carbonate;
In
water; acetone;
at 0 ℃;
for 2.5h;
|
59% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 1.5h;
|
59% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 1.5h;
|
59% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 1.5h;
|
59% |
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
59.7% |
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
59.7% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
|
59% |
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.333333h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
59.7% |
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
59.7% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 24h;
Inert atmosphere;
|
53% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 24h;
Inert atmosphere;
|
53% |
|
With
sodium carbonate;
|
48% |
|
With
pyridine;
In
tetrahydrofuran;
at 20 ℃;
Cooling with ice;
|
46.7% |
|
With
sodium hydrogencarbonate;
In
dichloromethane; water;
Ambient temperature;
|
38% |
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 1h;
|
28% |
|
With
sodium hydroxide;
at 0 ℃;
|
|
|
at 15 ℃;
zuletzt bei 50grad;
|
|
|
With
sodium hydroxide;
|
|
|
|
|
|
In
tetrahydrofuran;
|
|
|
In
dichloromethane;
|
12.91 g (94%) |
|
In
dichloromethane;
|
12.91 g (94%) |
|
In
dichloromethane;
at 0 - 20 ℃;
for 20h;
Inert atmosphere;
|
|
|
In
dichloromethane;
at 0 - 20 ℃;
for 21h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
|
|
|
With
triethylamine;
In
dichloromethane;
|
|
|
With
triethylamine;
at 0 ℃;
for 12h;
|
|
|
With
sodium carbonate;
In
dichloromethane;
at 0 ℃;
for 5h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 12h;
|
|
|
In
dichloromethane;
at 0 ℃;
for 12h;
Alkaline conditions;
|
|
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
95 g |
|
ethanolamine;
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
benzyl chloroformate;
In
dichloromethane;
at 0 ℃;
for 3h;
|
95 g |
-
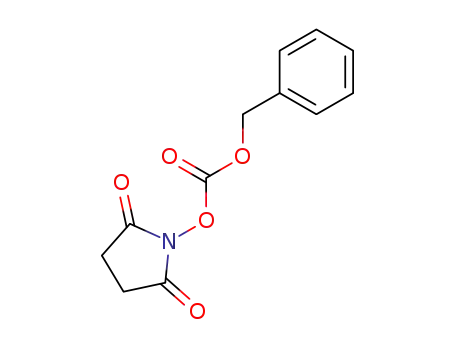
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-

-
141-43-5
ethanolamine

-

-
77987-49-6
benzyl 2-hydroxyethylcarbamate
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
water; acetone;
at 20 ℃;
for 4h;
|
100% |
|
With
TEA;
In
tetrahydrofuran;
for 5h;
|
92% |
|
In
1,4-dioxane; water;
for 12h;
|
89% |
|
With
1-butyl-3-methylimidazolium Tetrafluoroborate;
In
neat (no solvent);
at 25 ℃;
for 0.133333h;
chemoselective reaction;
|
86% |
|
In
ethyl acetate;
|
85% |
|
In
ethyl acetate;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
at 20 ℃;
Reagent/catalyst;
Solvent;
|
|
|
With
triethylamine;
In
tetrahydrofuran; dichloromethane;
at 20 ℃;
for 16h;
|
77987-49-6 Upstream products
-
141-43-5

ethanolamine
-
501-53-1

benzyl chloroformate
-
2768-56-1
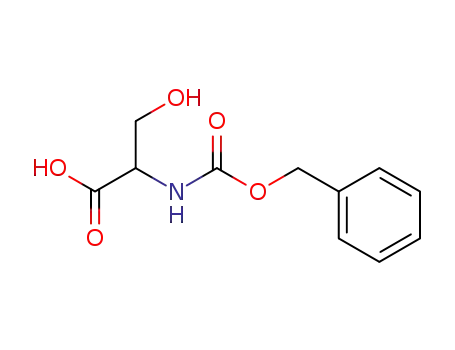
Z-DL-serine
-
55378-79-5
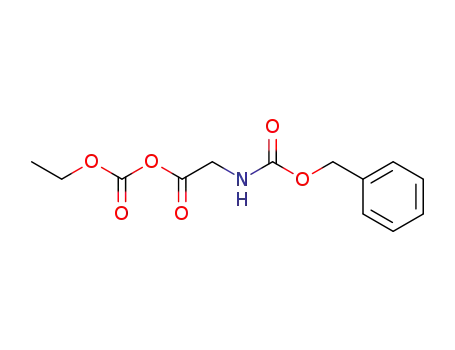
C13H15NO6
77987-49-6 Downstream products
-
103989-20-4
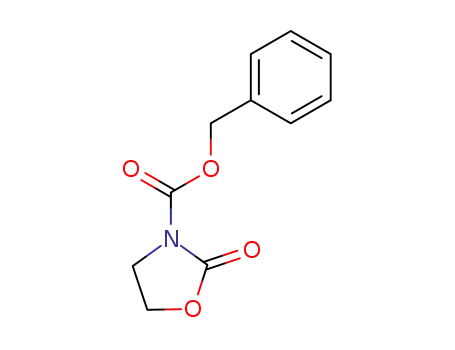
2-oxo-oxazolidine-3-carboxylic acid benzyl ester
-
33350-31-1
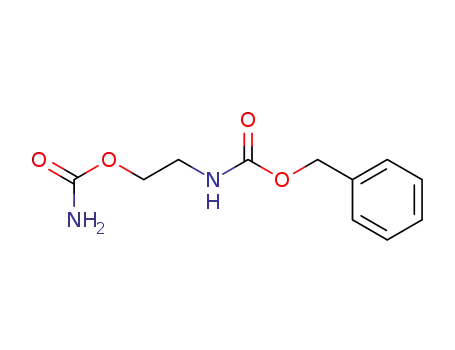
(2-carbamoyloxy-ethyl)-carbamic acid benzyl ester
-
6094-81-1
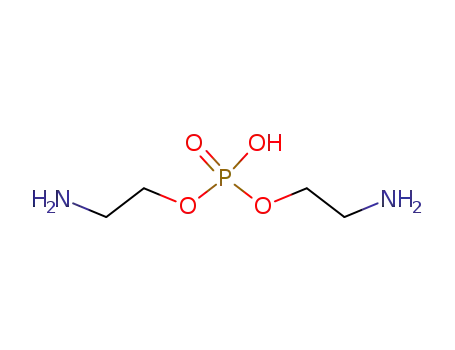
bis(2-aminoethanol) hydrogen phosphate
-
102313-50-8
(2-diphenoxyphosphoryloxy-ethyl)-carbamic acid benzyl ester
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
3-amino-1,2,3,4-tetrahydrocarbazole
CAS:61894-99-3
-
Dimethylol butanoic Acid
CAS:10097-02-6