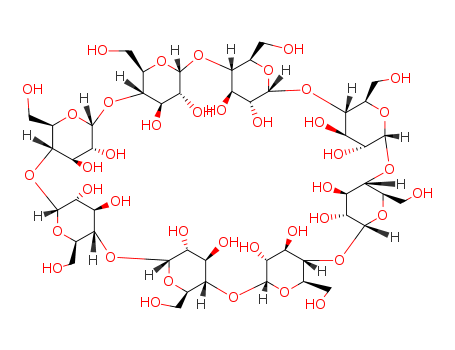
17465-86-0
- Product Name:Cyclooctapentylose
- Molecular Formula:C48H80O40
- Purity:99%
- Molecular Weight:1297.14
Product Details
pd_meltingpoint:267 °C
Appearance:white tpowder
Factory supply good quality Cyclooctapentylose 17465-86-0 with stock
- Molecular Formula:C48H80O40
- Molecular Weight:1297.14
- Appearance/Colour:white tpowder
- Melting Point:267 °C
- Refractive Index:1.591
- Boiling Point:845.2°C (rough estimate)
- PKA:11.68±0.70(Predicted)
- Flash Point:450℃
- PSA:633.20000
- Density:1.624 g/cm3
- LogP:-17.40640
Cyclooctapentylose(Cas 17465-86-0) Usage
|
Flammability and Explosibility |
Nonflammable |
|
Pharmaceutical Applications |
Cyclodextrins are ‘bucketlike’ or ‘conelike’ toroid molecules, with a rigid structure and a central cavity, the size of which varies according to the cyclodextrin type. The internal surface of the cavity is hydrophobic and the outside of the torus is hydrophilic; this is due to the arrangement of hydroxyl groups within the molecule. This arrangement permits the cyclodextrin to accommodate a guest molecule within the cavity, forming an inclusion complex.Cyclodextrins may be used to form inclusion complexes with a variety of drug molecules, resulting primarily in improvements to dissolution and bioavailability owing to enhanced solubility and improved chemical and physical stability.Cyclodextrin inclusion complexes have also been used to mask the unpleasant taste of active materials and to convert a liquid substance into a solid material. γ-cyclodextrin has the largest cavity and can be used to form inclusion complexes with large molecules;it has low toxicity and enhanced water solubility. In parenteral formulations, cyclodextrins have been used to produce stable and soluble preparations of drugs that would otherwise have been formulated using a nonaqueous solvent. In eye drop formulations, cyclodextrins form water-soluble complexes with lipophilic drugs such as corticosteroids. They have been shown to increase the water solubility of the drug; to enhance drug absorption into the eye; to improve aqueous stability; and to reduce local irritation.Cyclodextrins have also been used in the formulation of solutions,suppositories, and cosmetics. |
|
Safety |
Cyclodextrins are starch derivatives and are mainly used in oral and parenteral pharmaceutical formulations. They are also used in topical and ophthalmic formulations. Cyclodextrins are also used in cosmetics and food products, and are generally regarded as essentially nontoxic and nonirritant materials. However, when administered parenterally, β-cyclodextrin is not metabolized but accumulates in the kidneys as insoluble cholesterol complexes, resulting in severe nephrotoxicity. Cyclodextrin administered orally is metabolized by microflora in the colon, forming the metabolites maltodextrin, maltose, and glucose; these are themselves further metabolized before being finally excreted as carbon dioxide and water. Although a study published in 1957 suggested that orally administered cyclodextrins were highly toxic, more recent animal toxicity studies in rats and dogs have shown this not to be the case, and cyclodextrins are now approved for use in food products and orally administered pharmaceuticals in a number of countries. Cyclodextrins are not irritant to the skin and eyes, or upon inhalation. There is also no evidence to suggest that cyclodextrins are mutagenic or teratogenic. γ-Cyclodextrin LD50 (rat, IP): 4.6 g/kg LD50 (rat ,IV): 4.0 g/kg LD50 (rat, oral): 8.0 g/kg |
|
General Description |
Cyclodextrins belongs to the family of cyclic α-1,4-glucans and is made of glucose units. This molecule has a shape of a hollow cone. It possesses a hydrophilic external and hydrophobic internal surface, because of which cyclodextrins can form inclusion complexes. γ-Cyclodextrin is extensively used in food and pharmaceutical industries. Cyclodextrins are obtained by the enzymatic conversion of starch/starch derivatives by cyclodextrin glycosyltransferase. |
InChI:InChI=1/C48H80O40/c49-1-9-33-17(57)25(65)41(73-9)82-34-10(2-50)75-43(27(67)19(34)59)84-36-12(4-52)77-45(29(69)21(36)61)86-38-14(6-54)79-47(31(71)23(38)63)88-40-16(8-56)80-48(32(72)24(40)64)87-39-15(7-55)78-46(30(70)22(39)62)85-37-13(5-53)76-44(28(68)20(37)60)83-35-11(3-51)74-42(81-33)26(66)18(35)58/h9-72H,1-8H2/t9-,10-,11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m1/s1
17465-86-0 Relevant articles
Synthesis and supramolecular properties of regioisomers of mononaphthylallyl derivatives of v-cyclodextrin
Bláhová, Markéta,Filippov, Sergey K.,Ková?ik, Lubomír,Horsky, Ji?í,Hybelbauerová, Simona,Syrová, Zdenka,K?í?ek, Tomá?,Jind?ich, Jind?ich
, p. 2509 - 2520 (2017)
Monosubstituted derivatives of γ-cyclode...
A spectroscopic study of the inclusion of azulene by β- And γ-cyclodextrins
Abou-Zied, Osama K.
, p. 245 - 251 (2005)
The inclusion of azulene (AZ) inside the...
A novel cyclodextrin glucanotransferase from alkaliphilic Bacillus pseudalcaliphilus 20RF: Purification and properties
Atanasova, Nikolina,Kitayska, Tsvetina,Bojadjieva, Ivanka,Yankov, Dragomir,Tonkova, Alexandra
, p. 116 - 122 (2011)
A new cyclodextrin glucanotransferase (C...
Enzyme-mediated dynamic combinatorial chemistry allows out-of-equilibrium template-directed synthesis of macrocyclic oligosaccharides
Larsen, Dennis,Beeren, Sophie R.
, p. 9981 - 9987 (2019/11/14)
We show that the outcome of enzymatic re...
Altered product specificity of a cyclodextrin glycosyltransferase by molecular imprinting with cyclomaltododecaose
Kaulpiboon, Jarunee,Pongsawasdi, Piamsook,Zimmermann, Wolfgang
experimental part, p. 480 - 485 (2011/12/02)
Cyclodextrin glycosyltransferases (CGTas...
17465-86-0 Process route
-
soluble starch
-
soluble starch

-
-
7585-39-9
β‐cyclodextrin

-
-
17465-86-0
cyclomaltooctaose

-
-
10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
With
cyclodextrin glycosyltransferase from Paenibacillus Sp F8;
In
phosphate buffer;
pH=7.0;
Enzymatic reaction;
|
-
starch
-
9005-25-8
starch

-

-
7585-39-9
β‐cyclodextrin

-

-
17465-86-0
cyclomaltooctaose

-

-
10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
With
sodium phosphate buffer; Paenibacillus illinoisensis cyclodextrin glucanotransferase;
In
ethanol; water;
at 30 ℃;
for 24h;
pH=7.0;
|
17465-86-0 Upstream products
-
77192-53-1
prostacyclin*γ-cyclodextrin
-
77164-52-4
C48H80O40*C21H34O5
-
87832-35-7
C48H80O40*C16H10
-
9005-25-8
starch
17465-86-0 Downstream products
-
89239-13-4
C61H88N2O41
-
90510-11-5
C48H80O40*C19H31NO
-
98928-66-6
C48H80O40*C14H14N3O3S(1-)*Na(1+)
-
129371-88-6
octakis(3-O-benzyl-2,6-di-O-methyl)cyclomaltooctaose
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
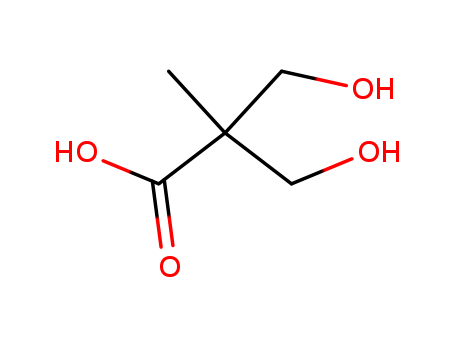
Dimethylolpropionic Acid
CAS:4767-03-7
-
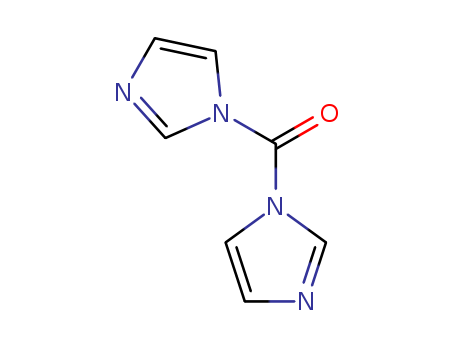
1,1'-Carbonyldiimidazole
CAS:530-62-1