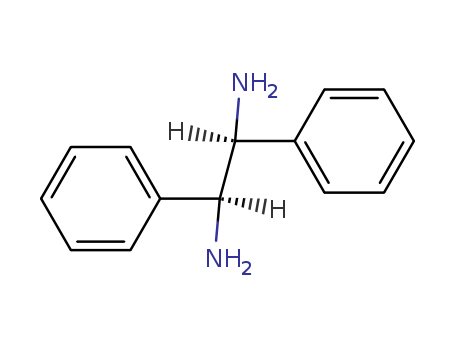
29841-69-8
- Product Name:(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine
- Molecular Formula:C14H16N2
- Purity:99%
- Molecular Weight:212.294
Product Details
pd_meltingpoint:83-85 °C(lit.)
Appearance:white to light yellow crystal powder
Top Quality Chinese Manufacturer supply 29841-69-8 (1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine
- Molecular Formula:C14H16N2
- Molecular Weight:212.294
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:3.48E-05mmHg at 25°C
- Melting Point:83-85 °C(lit.)
- Refractive Index:-103 ° (C=1, EtOH)
- Boiling Point:353.9 °C at 760 mmHg
- PKA:9.78±0.10(Predicted)
- Flash Point:199.9 °C
- PSA:52.04000
- Density:1.106 g/cm3
- LogP:3.78700
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine(Cas 29841-69-8) Usage
|
General Description |
1,2-Diphenylethylenediamine is a chiral molecule, generally used as a chiral resolving agent and as a precursor of the chiral auxiliary. It is also used as a chiral solvating agent in NMR study. |
InChI:InChI=1/C14H16N2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H,15-16H2/t13-,14-/m0/s1
29841-69-8 Relevant articles
Optical resolution of racemic stilbenediamine using N*-chiral ortho-palladated matrix
Dunina,Kuz'mina,Parfyonov,Griskin
, p. 183 - 194 (1999)
Optical resolution of racemic stilbenedi...
Stereospecific co-crystallization of reactant and palladium complex in the resolution of stilbendiamine using N*-chiral ortho-palladated matrix
Dunina,Kuz'mina,Parfyonov,Grishin
, p. 1917 - 1921 (1998)
Resolution of racemic 1,2-diphenyl-1,2-e...
Improved Optical Resolution of (+/-)-1,2-Diphenylethylenediamine
Saigo, Kazuhiko,Kubota, Naomi,Takebayashi, Shoko,Hasegawa, Masaki
, p. 931 - 932 (1986)
(+/-)-1,2-Diphenylethylenediamine (DPEDA...
Asymmetric synthesis X IV: TiCl(Oipr)3 - promoted asymmetric coupling reaction of d-camphor ketimine anion
Yaozhong,Wenhao,Jingen,Giulan
, p. 1755 - 1761 (1991)
Optically active 1,2-Diphenylethylenedia...
Enantioselective Reductive Coupling of Imines Templated by Chiral Diboron
Chen, Dongping,Li, Kaidi,Tang, Wenjun,Xu, Guangqing,Xu, Ronghua,Zhou, Mingkang
, p. 10337 - 10342 (2020/07/04)
We herein report a general, practical, a...
Diboron glycol ester as well as preparation method, intermediate and application thereof
-
, (2020/08/02)
The invention discloses diboron glycol e...
Mechanistic studies inform design of improved Ti(salen) catalysts for enantioselective [3 + 2] cycloaddition
Robinson, Sophia G.,Wu, Xiangyu,Jiang, Binyang,Sigman, Matthew S.,Lin, Song
supporting information, p. 18471 - 18482 (2020/11/17)
Ti(salen) complexes catalyze the asymmet...
Enantio- and Diastereoselective Nitro-Mannich Reaction of α-Aryl Nitromethanes with Amidosulfones Catalyzed by Phase-Transfer Catalysts
Lu, Ning,Li, Ruxu,Wei, Zhonglin,Cao, Jungang,Liang, Dapeng,Lin, Yingjie,Duan, Haifeng
, p. 4668 - 4676 (2017/05/12)
A high-yield, highly diastereo- and enan...
29841-69-8 Process route
-
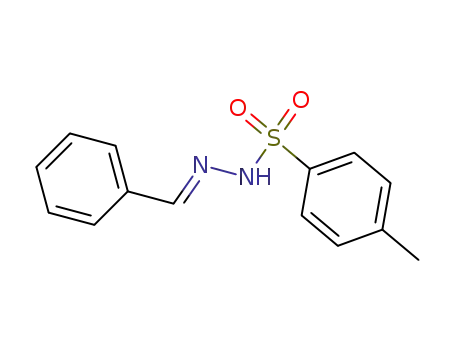
-
1666-17-7
(E)-N′-benzylidene-4-methylbenzenesulfonohydrazide

-

-
951-87-1,5700-60-7,16635-95-3,29841-69-8,35132-20-8,107133-00-6
meso-1,2-diphenyl-1,2-diaminoethane

-
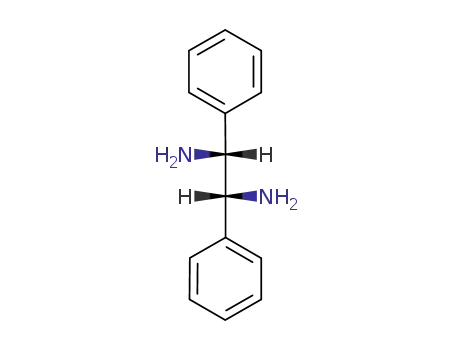
-
951-87-1,5700-60-7,16635-95-3,29841-69-8,35132-20-8,107133-00-6
rac-1,2-diphenylethylene-1,2-diamine

-
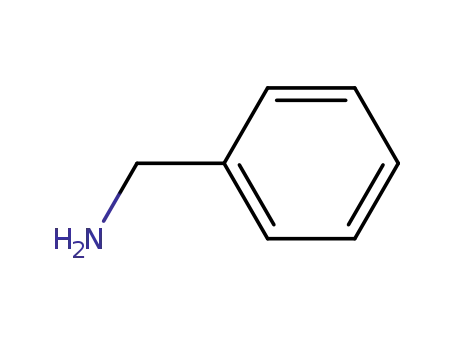
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid; zinc;
In
acetonitrile;
at 25 ℃;
for 8h;
|
31% |
|
With
titanium tetrachloride; zinc;
In
tetrahydrofuran;
at 25 ℃;
for 8h;
|
18% |
-

-
1666-17-7
(E)-N′-benzylidene-4-methylbenzenesulfonohydrazide

-

-
951-87-1,5700-60-7,16635-95-3,29841-69-8,35132-20-8,107133-00-6
meso-1,2-diphenyl-1,2-diaminoethane

-

-
951-87-1,5700-60-7,16635-95-3,29841-69-8,35132-20-8,107133-00-6
rac-1,2-diphenylethylene-1,2-diamine

-
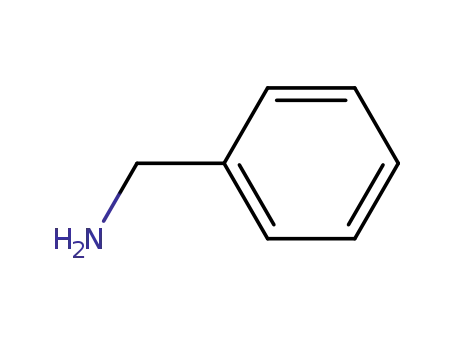
-
100-46-9
benzylamine
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid; zinc;
In
acetonitrile;
at 25 ℃;
for 8h;
|
31% |
|
With
titanium tetrachloride; zinc;
In
tetrahydrofuran;
at 25 ℃;
for 8h;
|
18% |
29841-69-8 Upstream products
-
951-87-1

rac-1,2-diphenylethylene-1,2-diamine
-
132486-62-5
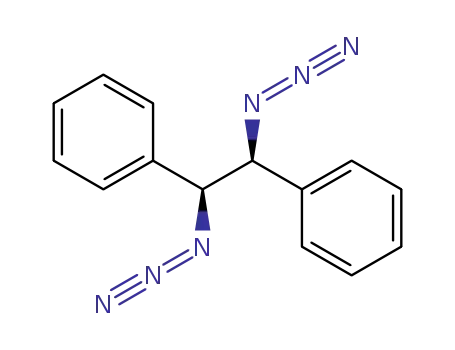
(+)-(1S,2S)-1,2-diphenylethane-1,2-diazide
-
137359-92-3
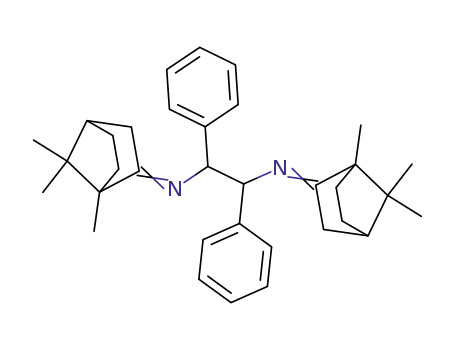
1,2-Diphenyl-N,N'-bis-[1,7,7-trimethyl-bicyclo[2.2.1]hept-(2E)-ylidene]-ethane-1,2-diamine
-
23873-81-6
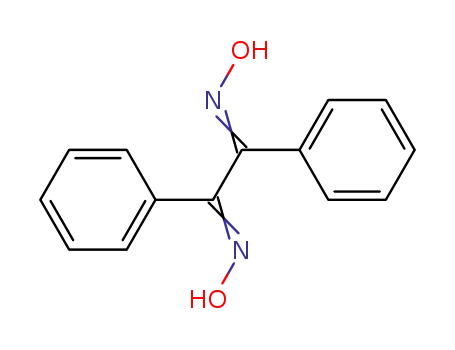
benzildioxime
29841-69-8 Downstream products
-
31819-61-1
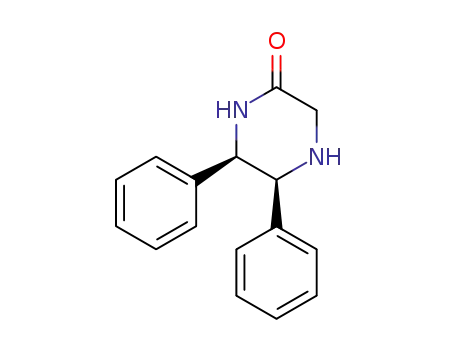
cis-5,6-diphenyl-piperazin-2-one
-
142819-75-8
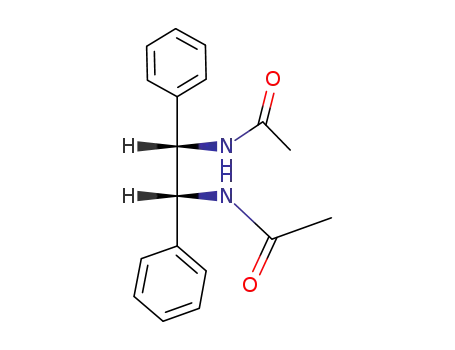
meso-N,N'-diacetyl-1,2-diamino-1,2-diphenylethane
-
642-04-6
2,3,5,6-tetraphenylpyrazine
-
51922-39-5
meso-N,N'-((E,E)-dibenzylidene)-bibenzyl-α,α'-diyldiamine
Relevant Products
-
1-Boc-3-Piperidinone
CAS:98977-36-7
-
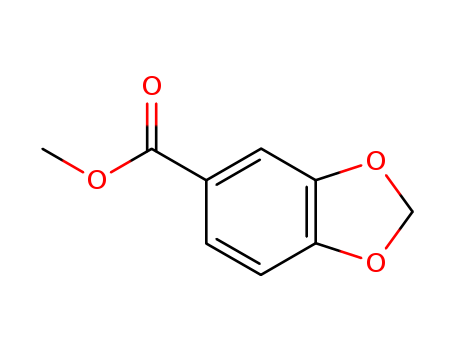
methyl 1,3-benzodioxole-5-carboxylate
CAS:326-56-7
-
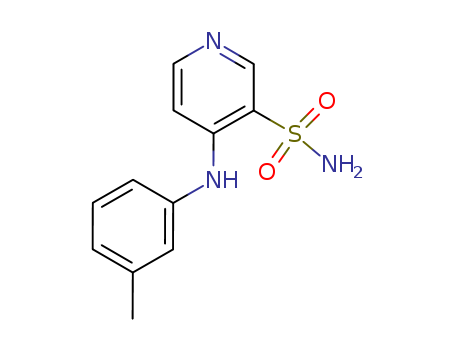
4-(3'-Methylphenyl)amino-3-pyridinesulfonamide
CAS:72811-73-5